SitoC7A-modified lipid nanoparticles for integrated mRNA delivery and targeted STING activation
IF 11.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
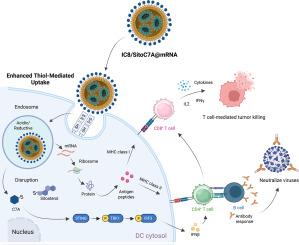
Messenger RNA (mRNA) vaccine efficacy requires delivery systems that maximize antigen expression while precisely modulating innate immune activation. However, many conventional adjuvants induce type I interferons that inadvertently suppress mRNA translation, compromising vaccine efficacy. To overcome this limitation, we engineered a multifunctional lipid nanoparticle (LNP) by partially substituting a fraction of the structural lipid cholesterol with SitoC7A - a conjugate of sitosterol and the STING (Stimulator of Interferon Genes) agonist (C7A) linked via a reducible disulfide bond. This single SitoC7A component serves dual roles: its disulfide bond markedly enhances LNP uptake by dendritic cells (DCs) and boosts mRNA translation, while its C7A moiety selectively triggers STING signaling within DCs, avoiding systemic interferon responses. This integrated design yielded robust DC maturation and superior antigen presentation. In murine models, SitoC7A-LNPs encapsulating SARS-CoV-2 mRNA elicited potent protective immunity, while tumor antigen-loaded LNPs significantly suppressed lymphoma progression. We introduce a principle for the novel LNP design wherein a single engineered lipid simultaneously provides structural support, enhances mRNA delivery and expression specifically in key immune cells, and delivers spatially controlled adjuvanticity creating a versatile and potent platform for next-generation mRNA vaccines.
sitoc7a修饰的脂质纳米颗粒用于整合mRNA传递和靶向STING激活
信使RNA (mRNA)疫苗的有效性需要递送系统最大化抗原表达,同时精确调节先天免疫激活。然而,许多传统佐剂诱导I型干扰素无意中抑制mRNA翻译,影响疫苗的功效。为了克服这一限制,我们设计了一种多功能脂质纳米颗粒(LNP),通过SitoC7A部分取代结构脂质胆固醇的一部分,SitoC7A是谷甾醇和干扰素基因刺激剂(STING)激动剂(C7A)的缀合物,通过可还原二硫键连接。这种单一的SitoC7A组分具有双重作用:其二硫键显著增强树突状细胞(dc)对LNP的摄取并促进mRNA翻译,而其C7A片段选择性地触发dc内的STING信号,避免系统性干扰素反应。这种集成设计产生了稳健的DC成熟和优越的抗原呈递。在小鼠模型中,包裹SARS-CoV-2 mRNA的SitoC7A-LNPs引发了有效的保护性免疫,而肿瘤抗原负载的LNPs显著抑制淋巴瘤进展。我们介绍了一种新型LNP设计的原理,其中单个工程脂质同时提供结构支持,增强mRNA在关键免疫细胞中的特异性传递和表达,并提供空间控制的佐剂性,为下一代mRNA疫苗创造了一个多功能和有效的平台。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


