Safety and efficacy of KRAS antisense oligonucleotides and RIG-I agonists delivered by extracellular vesicles for pancreatic cancer peritoneal metastasis treatment
IF 11.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Pancreatic ductal adenocarcinoma (PDAC) often metastasizes to the peritoneum and is highly resistant to treatments due to its immunosuppressive microenvironment. In this study, we evaluate the safety and efficacy of a novel therapeutic strategy that combines KRAS-targeting antisense oligonucleotides (ASOs) with immunomodulatory RNA (immRNA), a RIG-I agonist, both delivered by extracellular vesicles (EVs), in preclinical models using PDAC patient-derived organoids and mice bearing PDAC peritoneal metastasis. Our data demonstrate that the combination of KRAS ASO and immRNA synergistically activates anti-tumor immune responses. EV-mediated co-delivery of both agents significantly inhibits tumor growth, reduces peritoneal metastasis, and markedly prolongs overall survival through the induction of immunologic cancer cell death. Importantly, this combination therapy is well-tolerated in non-human primates, with no observable changes in physical condition or behavior, blood parameters, or organ histology. These findings suggest that EV-delivered KRAS ASO and immRNA is a safe and potent therapeutic approach for treating PDAC and its peritoneal metastasis, positioning it as a promising strategy for future clinical advancement.
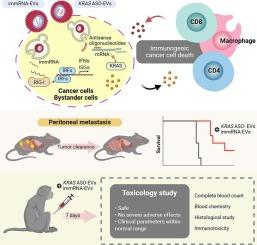
细胞外囊泡递送KRAS反义寡核苷酸和rig - 1激动剂治疗胰腺癌腹膜转移的安全性和有效性
胰腺导管腺癌(PDAC)经常转移到腹膜,由于其免疫抑制微环境,对治疗具有高度耐药性。在这项研究中,我们评估了一种新的治疗策略的安全性和有效性,该策略将靶向kras的反义寡核苷酸(ASOs)与免疫调节RNA (immRNA)(一种rig - 1激动剂)结合,两者都通过细胞外囊泡(ev)传递,在临床前模型中使用PDAC患者来源的类器官和患有PDAC腹膜转移的小鼠。我们的数据表明,KRAS、ASO和immRNA的联合可协同激活抗肿瘤免疫反应。ev介导的两种药物共递送可显著抑制肿瘤生长,减少腹膜转移,并通过诱导免疫癌细胞死亡显着延长总生存期。重要的是,这种联合疗法在非人类灵长类动物中耐受性良好,在身体状况或行为、血液参数或器官组织学上没有可观察到的变化。这些研究结果表明,ev递送KRAS ASO和immRNA是治疗PDAC及其腹膜转移的一种安全有效的治疗方法,将其定位为未来临床进展的有希望的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


