Time-resolved reprogramming of single somatic cells into totipotent states during plant regeneration
IF 42.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Totipotency enables single cells to regenerate an organism, yet how differentiated somatic cells reacquire this potential remains unclear. Here, we show that LEAFY COTYLEDON2 (LEC2) reprograms SPEECHLESS (SPCH)-expressing meristemoid mother cells (MMCs) away from stomatal-lineage progression, driving their conversion into totipotent somatic embryo founder cells (SEFCs) in Arabidopsis cotyledons. Using time-course live imaging, single-nucleus RNA sequencing (snRNA-seq), and spatial laser capture microdissection combined with RNA sequencing (LCM-RNA-seq), we uncover a lineage bifurcation point where MMC derivatives either commit to guard cells or transition into a guard mother cell (GMC)-auxin intermediate, an auxin-enriched state that enables transcriptional reprogramming and embryonic gene activation. LEC2 and SPCH cooperatively activate TRYPTOPHAN AMINOTRANSFERASE OF ARABIDOPSIS 1 (TAA1) and YUCCA4 (YUC4), establishing a local auxin biosynthesis circuit essential for SEFC specification. Genetic and promoter analyses confirm MMCs as the origin of somatic embryos, with TAA1/YUC-mediated auxin production indispensable for totipotency and embryogenesis. These findings define an auxin-driven, transcriptionally regulated trajectory linking stomatal progenitors to somatic embryogenesis, revealing a direct route that advances mechanistic understanding of plant regenerative plasticity.
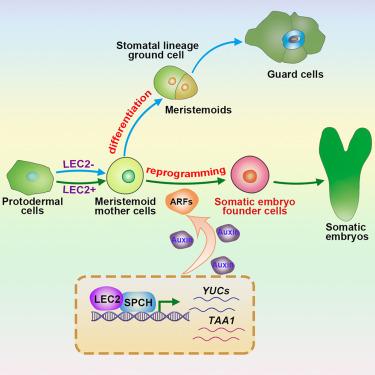
植物再生过程中单个体细胞进入全能性状态的时间分辨重编程
全能性使单个细胞能够再生生物体,但分化的体细胞如何重新获得这种潜力尚不清楚。本研究表明,在拟南芥子叶中,叶状子叶don2 (le2)重编程表达无语(SPCH)的分生组织母细胞(MMCs),使其远离气孔谱系的进展,从而驱动其转化为全能体细胞胚胎建立细胞(sefc)。利用实时成像、单核RNA测序(snRNA-seq)和空间激光捕获显微解剖结合RNA测序(LCM-RNA-seq),我们发现了一个谱系分叉点,MMC衍生物要么参与保护细胞,要么转变为保护母细胞(GMC)-生长素中间体,一种生长素富集状态,能够实现转录重编程和胚胎基因激活。LEC2和SPCH协同激活拟南芥1 (TAA1)和YUCCA4 (YUC4)的色氨酸氨基转移酶,建立了SEFC规范所必需的局部生长素生物合成回路。遗传和启动子分析证实mmc是体细胞胚胎的起源,TAA1/ yuc介导的生长素的产生对于全能性和胚胎发生是必不可少的。这些发现定义了生长素驱动的、转录调控的将气孔祖细胞与体细胞胚胎发生联系起来的轨迹,揭示了促进植物再生可塑性机制理解的直接途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell
生物-生化与分子生物学
CiteScore
110.00
自引率
0.80%
发文量
396
审稿时长
2 months
期刊介绍:
Cells is an international, peer-reviewed, open access journal that focuses on cell biology, molecular biology, and biophysics. It is affiliated with several societies, including the Spanish Society for Biochemistry and Molecular Biology (SEBBM), Nordic Autophagy Society (NAS), Spanish Society of Hematology and Hemotherapy (SEHH), and Society for Regenerative Medicine (Russian Federation) (RPO).
The journal publishes research findings of significant importance in various areas of experimental biology, such as cell biology, molecular biology, neuroscience, immunology, virology, microbiology, cancer, human genetics, systems biology, signaling, and disease mechanisms and therapeutics. The primary criterion for considering papers is whether the results contribute to significant conceptual advances or raise thought-provoking questions and hypotheses related to interesting and important biological inquiries.
In addition to primary research articles presented in four formats, Cells also features review and opinion articles in its "leading edge" section, discussing recent research advancements and topics of interest to its wide readership.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


