Replisome passage through the cohesin ring
IF 42.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Following eukaryotic genome replication, the ring-shaped cohesin complex embraces the two newly synthesized sister chromatids, enabling their faithful segregation during cell divisions. Replisome passage through cohesin rings has been envisioned as a fail-safe mechanism that ensures co-entrapment of replication products—whether replisomes can indeed pass through cohesin rings remains unknown. Here, we use biochemical reconstitution and single-molecule fluorescence microscopy to directly visualize replisome-cohesin encounters. We find that the translocating eukaryotic replicative Cdc45-Mcm2-7-GINS (CMG) helicase, unlike other obstacles of similar size, readily passes through cohesin rings. Fully reconstituted replisomes also pass cohesin rings to leave both replication products trapped inside. Replisome passage is primarily aided by DNA polymerases α and ε, a finding that necessitates re-evaluation of canonical cohesion establishment factor roles. Our findings demonstrate the existence of a simple mechanism that links genome replication with chromosome segregation: replisome passage through cohesin rings.
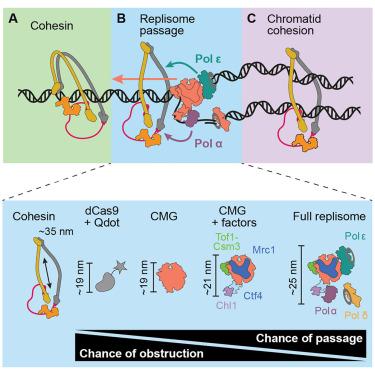
复制体通过内聚环
在真核生物基因组复制之后,环状内聚蛋白复合体包围了两个新合成的姐妹染色单体,使它们在细胞分裂期间能够忠实地分离。复制体通过内聚环被设想为一种确保复制产物共捕获的故障安全机制——复制体是否确实可以通过内聚环仍然未知。在这里,我们使用生化重构和单分子荧光显微镜来直接观察复制体-内聚蛋白的相遇。我们发现易位的真核生物复制性Cdc45-Mcm2-7-GINS (CMG)解旋酶与其他类似大小的障碍不同,它很容易通过内聚环。完全重组的复制体也通过内聚环,使两个复制产物都被困在里面。复制体的传代主要由DNA聚合酶α和ε辅助,这一发现需要重新评估典型内聚建立因子的作用。我们的研究结果表明,存在一种简单的机制,将基因组复制与染色体分离联系起来:复制体通过内聚环传递。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell
生物-生化与分子生物学
CiteScore
110.00
自引率
0.80%
发文量
396
审稿时长
2 months
期刊介绍:
Cells is an international, peer-reviewed, open access journal that focuses on cell biology, molecular biology, and biophysics. It is affiliated with several societies, including the Spanish Society for Biochemistry and Molecular Biology (SEBBM), Nordic Autophagy Society (NAS), Spanish Society of Hematology and Hemotherapy (SEHH), and Society for Regenerative Medicine (Russian Federation) (RPO).
The journal publishes research findings of significant importance in various areas of experimental biology, such as cell biology, molecular biology, neuroscience, immunology, virology, microbiology, cancer, human genetics, systems biology, signaling, and disease mechanisms and therapeutics. The primary criterion for considering papers is whether the results contribute to significant conceptual advances or raise thought-provoking questions and hypotheses related to interesting and important biological inquiries.
In addition to primary research articles presented in four formats, Cells also features review and opinion articles in its "leading edge" section, discussing recent research advancements and topics of interest to its wide readership.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


