Signaling reprogramming via Stat3 activation unravels high-fidelity human post-implantation embryo modeling
IF 20.4
1区 医学
Q1 CELL & TISSUE ENGINEERING
引用次数: 0
Abstract
Human embryo models hold great promise for advancing medicine, but current systems lack efficiency and fidelity in replicating post-implantation stages. Here, we investigate whether STAT3 activation can reprogram pluripotent stem cells (PSCs) into early fates that self-organize into embryo models. Using a medium enhancing STAT3 activity (SAM), PSCs reprogram within 60 h into hypoblast, trophectoderm, naive epiblast, and extraembryonic mesoderm. Dissociating SAM-treated PSCs at 60–120 h, followed by 3D culture, results in dynamic development of post-implantation embryo-like structures with up to 52.41% ± 8.92% efficiency. Resulting day 6 examples resemble Carnegie stages 5 (CS5) to 7 (CS7) embryos, exhibiting bilaminar disc structure with epiblast and yolk sac, amniotic cavity, mesenchyme, chorionic cavity, and trophoblast. Notably, CS6/7-like examples exhibit gastrulation, including the formation and correct positioning of primitive streak, epithelial-to-mesenchymal transition, mesoderm, and definitive endoderm. The STAT3-mediated embryo model also closely aligns molecularly with CS6/7 embryo references and represents a state-of-the-art platform for advancing human embryogenesis research.
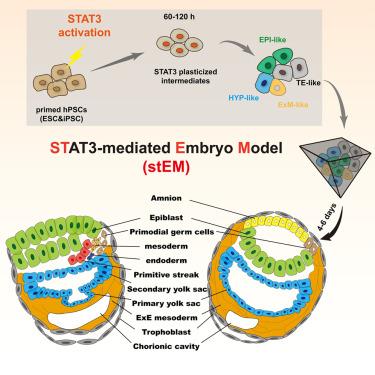
通过Stat3激活的信号重编程揭示了高保真的人类植入后胚胎建模
人类胚胎模型在推进医学方面有着巨大的希望,但目前的系统在复制植入后阶段缺乏效率和保真度。在这里,我们研究STAT3激活是否可以重编程多能干细胞(PSCs)进入自组织成胚胎模型的早期命运。使用增强STAT3活性(SAM)的培养基,PSCs在60小时内重编程为下胚层、滋养外胚层、原始外胚层和胚外中胚层。在60-120 h解离经过sam处理的PSCs,然后进行3D培养,可获得植入后胚胎样结构的动态发育,效率高达52.41%±8.92%。第6天的样品类似于卡内基5期(CS5)至7期(CS7)胚胎,表现出双层盘状结构,包括外胚层和卵黄囊、羊膜腔、间充质、绒毛膜腔和滋养细胞。值得注意的是,cs6 /7样样本显示原肠胚形成,包括原始条纹的形成和正确定位、上皮向间质转变、中胚层和终胚层。stat3介导的胚胎模型在分子上也与CS6/7胚胎文献密切一致,代表了推进人类胚胎发生研究的最先进平台。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell stem cell
生物-细胞生物学
CiteScore
37.10
自引率
2.50%
发文量
151
审稿时长
42 days
期刊介绍:
Cell Stem Cell is a comprehensive journal covering the entire spectrum of stem cell biology. It encompasses various topics, including embryonic stem cells, pluripotency, germline stem cells, tissue-specific stem cells, differentiation, epigenetics, genomics, cancer stem cells, stem cell niches, disease models, nuclear transfer technology, bioengineering, drug discovery, in vivo imaging, therapeutic applications, regenerative medicine, clinical insights, research policies, ethical considerations, and technical innovations. The journal welcomes studies from any model system providing insights into stem cell biology, with a focus on human stem cells. It publishes research reports of significant importance, along with review and analysis articles covering diverse aspects of stem cell research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


