OGFOD1 enables AML chemo- and nutrient stress resistance by regulating protein synthesis
IF 30.9
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
Acute myeloid leukemia (AML) commonly relapses after initial chemotherapy response. We assessed metabolic adaptations in chemoresistant cells in vivo before overt relapse, identifying altered branched-chain amino acid (BCAA) levels in patient-derived xenografts (PDXs) and immunophenotypically identified leukemia stem cells from AML patients. Notably, this was associated with increased BCAA transporter expression with low BCAA catabolism. Restricting BCAAs further reduced chemoresistant AML cells, but relapse still occurred. Among the persisting cells, we found an unexpected increase in protein production. This was accompanied by elevated translation of 2-oxoglutarate- and iron-dependent oxygenase 1 (OGFOD1), a known ribosomal dioxygenase that adjusts the fidelity of tRNA anticodon pairing with coding mRNA. We found that OGFOD1 upregulates protein synthesis in AML, driving disease aggressiveness. Inhibiting OGFOD1 impaired translation processing, decreased protein synthesis and improved animal survival even with chemoresistant AML while sparing normal hematopoiesis. Leukemic cells can therefore persist despite the stress of chemotherapy and nutrient deprivation through adaptive control of translation. Targeting OGFOD1 may offer a distinctive, translation-modifying means of reducing the chemopersisting cells that drive relapse.
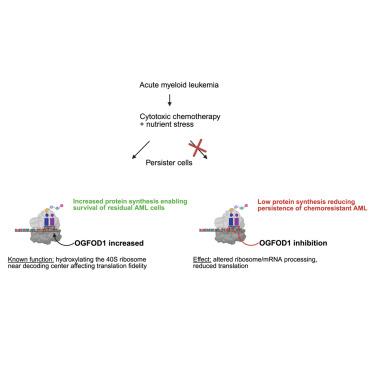
OGFOD1通过调节蛋白质合成使AML抵抗化学和营养胁迫
急性髓性白血病(AML)通常在初始化疗反应后复发。我们评估了化疗耐药细胞在明显复发前的体内代谢适应,鉴定了患者来源的异种移植物(PDXs)和AML患者免疫表型鉴定的白血病干细胞中支链氨基酸(BCAA)水平的改变。值得注意的是,这与BCAA转运蛋白表达增加和低BCAA分解代谢有关。限制BCAAs进一步减少了化疗耐药的AML细胞,但仍发生复发。在这些持续存在的细胞中,我们发现蛋白质产量出乎意料地增加了。这伴随着2-氧戊二酸和铁依赖性加氧酶1 (OGFOD1)的翻译升高,这是一种已知的核糖体双加氧酶,可调节tRNA反密码子与编码mRNA配对的保真度。我们发现,OGFOD1上调AML中的蛋白合成,推动疾病侵袭性。抑制OGFOD1会损害翻译加工,降低蛋白质合成,甚至在保留正常造血功能的情况下,也能改善化疗耐药AML的动物存活率。因此,尽管化疗和营养剥夺的压力,白血病细胞可以通过自适应控制翻译而持续存在。靶向OGFOD1可能提供一种独特的,翻译修饰的方法来减少导致复发的化学持续细胞。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell metabolism
生物-内分泌学与代谢
CiteScore
48.60
自引率
1.40%
发文量
173
审稿时长
2.5 months
期刊介绍:
Cell Metabolism is a top research journal established in 2005 that focuses on publishing original and impactful papers in the field of metabolic research.It covers a wide range of topics including diabetes, obesity, cardiovascular biology, aging and stress responses, circadian biology, and many others.
Cell Metabolism aims to contribute to the advancement of metabolic research by providing a platform for the publication and dissemination of high-quality research and thought-provoking articles.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


