Accelerating the catalytic conversion of lithium polysulfides in lithium sulfur batteries via NiV-LDH/CNT
IF 5.6
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
Abstract
To address the critical challenges of polysulfide shuttle effects and sluggish redox kinetics in lithium-sulfur batteries (LSBs), this study synthesizes a nickel-vanadium layered double hydroxide/carbon nanotube (NiV-LDH/CNT) composite cathode material through a one-step hydrothermal method, as efficient electrocatalysts for LSBs. SEM and BET tests show that the NiV-LDH/CNT composite material increase the specific surface area and exposes more active sites, providing space for sulfur storage and alleviating sulfur volume expansion. Meanwhile, theoretical calculations indicate that NiV-LDH/CNT lowers the reaction energy barrier for lithium polysulfides (LiPSs), effectively accelerating the redox reactions of LiPSs. As a result, the NiV-LDH/CNT electrode demonstrates stable cycling performance, achieving a high initial specific capacity of 1091.7 mAh g-1 at 1.0 C with a capacity decay rate of only 0.07% per cycle in 800 cycles. Even under harsh conditions (with a sulfur loading of 7.1 mg cm-2 and an E/S ratio of 6.0 μL mg-1), an initial specific capacity of 1000.9 mAh g-1 can still be achieved. This design overcomes the limitations of traditional carbon-based cathodes reliant on physical confinement alone, offering a novel strategy to advance the practical implementation of high-energy-density LSBs.
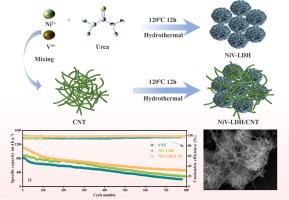
NiV-LDH/CNT催化硫锂电池中锂多硫化物的加速转化
为了解决锂硫电池(LSBs)中多硫化物穿梭效应和缓慢的氧化还原动力学的关键挑战,本研究通过一步水热法合成了镍钒层状双氢氧化物/碳纳米管(NiV-LDH/CNT)复合正极材料,作为LSBs的高效电催化剂。SEM和BET测试表明,NiV-LDH/CNT复合材料增加了比表面积,暴露了更多的活性位点,为硫的储存提供了空间,减轻了硫的体积膨胀。同时,理论计算表明,NiV-LDH/CNT降低了锂多硫化物(LiPSs)的反应能垒,有效地加速了LiPSs的氧化还原反应。结果表明,NiV-LDH/CNT电极表现出稳定的循环性能,在1.0℃下达到1091.7 mAh g-1的高初始比容量,在800次循环中,每次循环的容量衰减率仅为0.07%。即使在恶劣条件下(含硫量为7.1 mg cm-2, E/S比为6.0 μL mg-1),仍可实现1000.9 mAh g-1的初始比容量。该设计克服了传统碳基阴极仅依赖物理约束的局限性,为推进高能量密度lsdb的实际实施提供了一种新的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


