Graphene Foam Interdigitated Microband Arrays: [Fe(CN)6]3-/4- Generator-Collector Redox Processes and Prussian Blue Catalyst Attachment
IF 5.6
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
Abstract
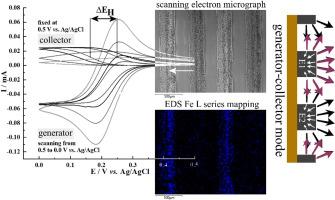
Graphene foam based interdigitated microband array electrodes with typically 400 μm width and 150 μm inter-electrode gap (10 anodes and 10 cathodes, each 5.8 mm long; active array area 63.5 mm2) were produced on polyimide substrates and investigated for electrochemical performance. The one-electron reversible aqueous Fe(CN)63-/4- redox system is employed in the presence and in the absence of 0.1 M KNO3 supporting electrolyte. Voltammetric responses on graphene foam suggest essentially reversible electron transfer under diffusion control with solution resistivity/migration contributing to the peak-to-peak separation especially in the absence of added electrolyte. Under bipotentiostatic control, generator-collector feedback current signals are recorded with a signal hysteresis (affected by solution resistivity) consistent with a relatively wide inter-electrode gap. With a positive potential bias during repetitive cyclic voltammetry, Prussian blue deposits/electrocatalysts form on the graphene foam. Generator-collector processes for combined oxygen reduction-hydrogen peroxide oxidation are observed.
石墨烯泡沫交错微带阵列:[Fe(CN)6]3-/4-发生器-收集器氧化还原过程和普鲁士蓝催化剂吸附
在聚酰亚胺衬底上制备了宽度为400 μm、电极间隙为150 μm(10个阳极和10个阴极,每个5.8 mm长,有源阵列面积为63.5 mm2)的石墨烯泡沫基交叉指状微带阵列电极,并对其电化学性能进行了研究。在0.1 M KNO3支持电解质存在和不存在的情况下,采用单电子可逆的Fe(CN)63-/4-水溶液氧化还原体系。石墨烯泡沫上的伏安响应表明,扩散控制下的电子转移本质上是可逆的,溶液电阻率/迁移有助于峰间分离,特别是在没有添加电解质的情况下。在双恒电位控制下,发生器-集电极反馈电流信号被记录下来,信号迟滞(受溶液电阻率影响)与相对宽的电极间隙一致。在重复循环伏安法中,由于电位偏置为正,普鲁士蓝沉积物/电催化剂会在石墨烯泡沫上形成。观察了联合氧还原-过氧化氢氧化的发生器-收集器过程。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


