High-performance liquid chromatography method for simultaneous determination of the degradation products of metformin hydrochloride and vildagliptin
IF 4.7
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
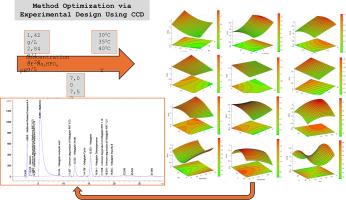
With the growing production of tablets containing two or more active pharmaceutical ingredients, analytical methods must align with this patient-friendly trend. The development and optimization of a gradient HPLC method for the simultaneous determination of degradation products of metformin and vildagliptin are performed using Full Factorial Design, which allows the significance check of selected factors. Central Composite Design optimizes the method to achieve the desired resolution between critical peak pairs.
During the optimization process, the pH and salt concentration in the buffer solution were adjusted, while the oven temperature remained unchanged compared to the initial chromatographic conditions. Separation was achieved using a reversed-phase column. Optimization improved the resolutions between all four critical peak pairs, especially for the critical pair related to vildagliptin. The response surface graphs were used to identify optimal experimental conditions, aligning fully with the optimized method. The aim of this paper is to present the method development and prove that it suits the intended purpose.
高效液相色谱法同时测定盐酸二甲双胍和维格列汀的降解产物。
随着含有两种或两种以上活性药物成分的片剂生产的增长,分析方法必须与这种对患者友好的趋势保持一致。采用全因子设计,建立并优化了同时测定二甲双胍和维格列汀降解产物的梯度高效液相色谱法,对所选因素进行显著性检验。中心复合设计优化的方法,以达到所需的分辨率之间的关键峰对。优化过程中,调整缓冲液的pH和盐浓度,同时保持烘箱温度与初始色谱条件不变。采用反相柱实现分离。优化提高了所有四个关键峰对之间的分辨率,特别是与维格列汀相关的关键峰对。利用响应面图确定最佳实验条件,与优化后的方法完全吻合。本文的目的是介绍方法的发展,并证明它符合预期的目的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


