Role of SIX5-mediated EXO1 overexpression in driving glioblastoma progression: Insights into tumor cell migration and angiogenesis
IF 2.6
4区 医学
Q3 NEUROSCIENCES
引用次数: 0
Abstract
Glioblastoma multiforme (GBM) is the most aggressive primary brain tumor, characterized by high recurrence and resistance to standard treatments, underscoring the pressing need for more effective and targeted therapeutic strategies. This study delved into the roles of sine oculis homeobox homolog 5 (SIX5) and exonuclease 1 (EXO1) genes in GBM pathogenesis and their potential as therapeutic targets. Through a comprehensive approach, utilizing bioinformatics analysis and experimental assays, we uncovered crucial insights. Firstly, high expression of EXO1 within GBM tissues was identified. Functional analyses following EXO1 knockdown revealed significant suppression of GBM cell viability, proliferation, migration, invasion, and induced DNA fragmentation. Notably, the suppression of EXO1 effectively hindered tumor growth in a subcutaneous xenograft model. Furthermore, our investigation highlighted SIX5 as an upstream regulator of EXO1. We elucidated the transcriptional relationship within the SIX5/EXO1 axis in GBM cells through ChIP experiments and dual-luciferase reporter gene assays. Intriguingly, the downregulation of SIX5 exhibited inhibitory effects on GBM cell growth in vitro, which were partially reversed by the overexpression of EXO1. In conclusion, the interplay between SIX5 and EXO1 emerges as a critical axis driving GBM development, shedding light on potential mechanisms for targeted interventions in combating this aggressive malignancy.
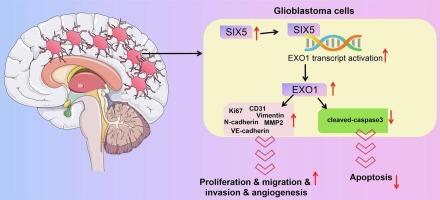
six5介导的EXO1过表达在驱动胶质母细胞瘤进展中的作用:肿瘤细胞迁移和血管生成的见解。
多形性胶质母细胞瘤(GBM)是最具侵袭性的原发性脑肿瘤,其特点是高复发和对标准治疗的抵抗,迫切需要更有效和更有针对性的治疗策略。本研究探讨了眼内同源盒同源物5 (SIX5)和外切酶1 (EXO1)基因在GBM发病机制中的作用及其作为治疗靶点的潜力。通过综合的方法,利用生物信息学分析和实验分析,我们发现了重要的见解。首先,在GBM组织中发现了EXO1的高表达。EXO1敲除后的功能分析显示,GBM细胞活力、增殖、迁移、侵袭和诱导的DNA片段化受到显著抑制。值得注意的是,在皮下异种移植模型中,EXO1的抑制有效地阻碍了肿瘤的生长。此外,我们的研究强调了SIX5是EXO1的上游调节因子。我们通过ChIP实验和双荧光素酶报告基因分析阐明了GBM细胞中SIX5/EXO1轴的转录关系。有趣的是,SIX5的下调对GBM细胞的体外生长表现出抑制作用,这种抑制作用部分被EXO1的过表达逆转。总之,SIX5和EXO1之间的相互作用是驱动GBM发展的关键轴,揭示了靶向干预对抗这种侵袭性恶性肿瘤的潜在机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Brain Research
医学-神经科学
CiteScore
5.90
自引率
3.40%
发文量
268
审稿时长
47 days
期刊介绍:
An international multidisciplinary journal devoted to fundamental research in the brain sciences.
Brain Research publishes papers reporting interdisciplinary investigations of nervous system structure and function that are of general interest to the international community of neuroscientists. As is evident from the journals name, its scope is broad, ranging from cellular and molecular studies through systems neuroscience, cognition and disease. Invited reviews are also published; suggestions for and inquiries about potential reviews are welcomed.
With the appearance of the final issue of the 2011 subscription, Vol. 67/1-2 (24 June 2011), Brain Research Reviews has ceased publication as a distinct journal separate from Brain Research. Review articles accepted for Brain Research are now published in that journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


