Lattice expansion in Ni3ZnC0.7@C weakening CO adsorption for efficient CO2 electroreduction
IF 14.9
1区 化学
Q1 Energy
引用次数: 0
Abstract
The metallic Ni catalyst suffers from strong binding with the *CO intermediate, resulting in poisoning of the catalyst surface. It is feasible to facilitate the generation of CO by alleviating the binding strength of the *CO intermediate on the Ni metal surface through a lattice expansion strategy. Here, Ni3ZnC0.7@C with lattice expansion was synthesized by co-doping with Zn and interstitial C through high-temperature pyrolysis. Structural characterization confirms that the lattice of Ni3ZnC0.7 expands by 5.47 % compared to Ni due to the co-doping of Zn and interstitial C. The Ni3ZnC0.7@C possesses excellent catalytic performance with Faradaic efficiency (FE) of CO exceeding 90 % over a wide potential range from −0.8 to −1.4 V versus reversible hydrogen electrode (vs. RHE) with a peak FECO of 96.6 % at −1.0 V vs. RHE. In membrane electrode assembly (MEA) testing, Ni3ZnC0.7@C achieves a FECO of 81.4 % at the industrial-level current density of 400 mA cm−2. In situ attenuated total reflection surface-enhanced infrared absorption spectroscopy (ATR-SEIRAS) and density functional theory (DFT) calculations reveal that the co-introduction of Zn and interstitial C in the Ni crystal can significantly promote the desorption of *CO intermediate, which facilitates the generation of CO. This study demonstrates a viable way for designing efficient transition metal catalysts for CO2 electroreduction through lattice strain engineering.
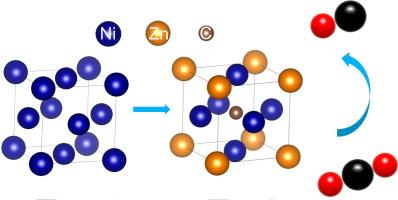
Ni3ZnC0.7@C中晶格膨胀削弱CO吸附,实现高效CO2电还原
金属镍催化剂与*CO中间体结合较强,导致催化剂表面中毒。通过晶格扩展策略减轻*CO中间体在Ni金属表面的结合强度,促进CO的生成是可行的。本文通过高温热解与Zn和间隙C共掺杂,合成了具有晶格扩展的Ni3ZnC0.7@C。结构表征证实,与Ni相比,由于Zn和间隙c的共掺杂,Ni3ZnC0.7的晶格膨胀了5.47%。Ni3ZnC0.7@C与可逆氢电极相比,在−0.8 ~−1.4 V的宽电位范围内,CO的法拉第效率(FE)超过90%,在−1.0 V时FECO峰值为96.6%。在膜电极组装(MEA)测试中,Ni3ZnC0.7@C在工业级电流密度为400 mA cm - 2时达到81.4%的FECO。原位衰减全反射表面增强红外吸收光谱(ATR-SEIRAS)和密度泛函理论(DFT)计算表明,在Ni晶体中引入Zn和间隙C可以显著促进*CO中间体的解吸,从而促进CO的生成。本研究为通过晶格应变工程设计高效的CO2电还原过渡金属催化剂提供了一条可行的途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


