Co-P covalent interaction strengthens electron relocation in Ce-O-Co for enhanced water oxidation via lattice oxygen mechanism
IF 22
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
Due to restrictive linear relationship between intermediates in adsorption evolution mechanism (AEM) of oxygen evolution reaction (OER), a higher overpotential must be overcome for conversion of *O to *OOH. Activating lattice oxygen mechanism (LOM) can address the AEM’s challenges by facilitating O-O coupling. However, via LOM pathway, extracting electrons from metal-oxygen (M−O) bonds usually results in structural instability. Herein, we propose an effective method to stabilize LOM-catalysts by combining cerium dioxide with cobalt phosphide (CeO2/CoP heterostructure). CeO2 acts as an electron buffer to retain/provide electrons at different stages, while CoP with strong Co-P covalency enhances structural/catalytic stability in strongly-alkaline conditions. CeO2/CoP exhibits an OER overpotential of only 225 mV at 10 mA cm−2. Initially, CeO2 acquires electrons from CoP to accelerate surface reconstruction and form high-valence Coδ+ (CoOOH). Then, CoOOH acquires electrons from the 4f-orbital of Ce through the Ce-O-Co interface, rather than from the M−O bond, thus ensuring OER stability. For water splitting, bifunctional CeO2/CoP-assembled electrolyzer requires a cell-voltage of only 1.54 V to achieve 10 mA cm−2 and retains 89.5 % of its activity after 72 h of cycling. It highlights that leveraging electron-buffering metal species can enhance the LOM stability, thereby enabling its application in bifunctional electrocatalysis.
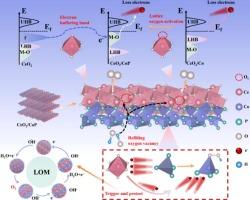
Co-P共价相互作用增强Ce-O-Co中的电子重定位,通过点阵氧机制增强水氧化
由于析氧反应(OER)的吸附演化机制(AEM)中中间体之间存在限制性线性关系,因此*O转化为*OOH必须克服较高的过电位。激活晶格氧机制(LOM)可以通过促进O-O耦合来解决AEM的挑战。然而,通过LOM途径,从金属-氧(M−O)键中提取电子通常会导致结构不稳定。本文提出了一种稳定lom催化剂的有效方法,即将二氧化铈与磷化钴(CeO2/CoP异质结构)结合。CeO2作为电子缓冲器在不同阶段保留/提供电子,而具有强Co-P共价的CoP在强碱性条件下增强了结构/催化稳定性。CeO2/CoP在10 mA cm−2时的OER过电位仅为225 mV。最初,CeO2从CoP获得电子,加速表面重构,形成高价Coδ+ (CoOOH)。然后,CoOOH通过Ce-O- co界面从Ce的4f轨道获得电子,而不是从M -O键获得电子,从而保证了OER稳定性。对于水分解,双功能CeO2/ cop组装电解槽只需要1.54 V的电池电压就可以达到10 mA cm - 2,并且在循环72小时后保持89.5%的活性。强调利用电子缓冲金属可以提高LOM的稳定性,从而使其在双功能电催化中的应用成为可能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Today
工程技术-材料科学:综合
CiteScore
36.30
自引率
1.20%
发文量
237
审稿时长
23 days
期刊介绍:
Materials Today is the leading journal in the Materials Today family, focusing on the latest and most impactful work in the materials science community. With a reputation for excellence in news and reviews, the journal has now expanded its coverage to include original research and aims to be at the forefront of the field.
We welcome comprehensive articles, short communications, and review articles from established leaders in the rapidly evolving fields of materials science and related disciplines. We strive to provide authors with rigorous peer review, fast publication, and maximum exposure for their work. While we only accept the most significant manuscripts, our speedy evaluation process ensures that there are no unnecessary publication delays.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


