Local structural distortion and energy gradient enhance lithium ionic conductivity in high-entropy oxide
IF 22
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
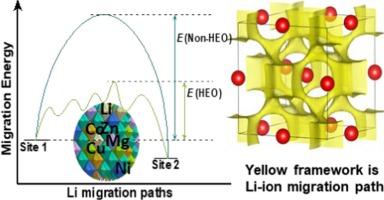
Li-rich disordered rock-salt oxides have been extensively studied as electrode materials for lithium-ion batteries, however, their diffusion of lithium ions relies on the presence of excess lithium-ion content (>54.5 atom% relative to total metal ions). An emerging high-entropy strategy can reduce the lithium-ion content and enhance lithium-ion conductivity in sodium superionic conductor (e.g. Li(Ti,Zr,Sn,Hf)2(PO4)3). However, the high ionic conductivity in Li-stuffed disordered rock-salt oxides with low lithium-ion content is generally attributed to its cocktail effect, and the underlying mechanisms remains unclear. Here, we develop a robust Li-poor disordered rock-salt high-entropy oxide, (MgCoNiCuZn)0.75Li0.25O (HEOLi) as an artificial solid electrolyte interphase coating layer to stabilize lithium metal anodes, achieving an impressive cycling stability of over 15000 h. We elucidate a cocktail effect of HEOLi arising from its disordered structure of HEOLi, with unique crystallographic local structural distortions, delocalized electron structure, and energy gradients, enabling high Li-ion conductivity. These energy gradients reduce the overall energy barrier and promote Li+ hopping through preferential pathways within the HEOLi. This work offers insight into the cocktail effect of high-entropy and the Li-ion conduction mechanism, facilitating the rational design of conductive high-entropy ceramics.
局部结构畸变和能量梯度增强了高熵氧化物中锂离子的电导率
富锂无序岩盐氧化物作为锂离子电池的电极材料已被广泛研究,然而,它们的锂离子扩散依赖于过量锂离子含量的存在(相对于总金属离子的54.5原子%)。一种新兴的高熵策略可以降低锂离子含量,提高锂离子在钠超离子导体(如Li(Ti,Zr,Sn,Hf)2(PO4)3)中的电导率。然而,低锂离子含量的锂填充无序岩盐氧化物的高离子电导率通常归因于其鸡尾酒效应,其潜在机制尚不清楚。在这里,我们开发了一种强大的贫锂无序岩盐高熵氧化物(MgCoNiCuZn)0.75Li0.25O (HEOLi)作为人造固体电解质间相涂层来稳定锂金属阳极,实现了超过15000小时的令人惊讶的循环稳定性。我们阐明了HEOLi的鸡尾酒效应,HEOLi具有独特的晶体局部结构扭曲,离域电子结构和能量梯度,使锂离子具有高导电性。这些能量梯度降低了整体能量势垒,促进Li+在HEOLi内通过优先途径跳变。本研究对高熵的鸡尾酒效应和锂离子的传导机理有了深入的认识,有助于导电高熵陶瓷的合理设计。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Today
工程技术-材料科学:综合
CiteScore
36.30
自引率
1.20%
发文量
237
审稿时长
23 days
期刊介绍:
Materials Today is the leading journal in the Materials Today family, focusing on the latest and most impactful work in the materials science community. With a reputation for excellence in news and reviews, the journal has now expanded its coverage to include original research and aims to be at the forefront of the field.
We welcome comprehensive articles, short communications, and review articles from established leaders in the rapidly evolving fields of materials science and related disciplines. We strive to provide authors with rigorous peer review, fast publication, and maximum exposure for their work. While we only accept the most significant manuscripts, our speedy evaluation process ensures that there are no unnecessary publication delays.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


