Green efficient dual-strategy for critical metal recovery from spent LIBs: Nipa palm shell-derived cellulose and [C4H9NH3][Cyanex 272] ionic liquid extraction
IF 4.2
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
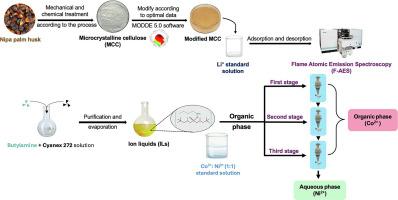
Green and environmentally friendly natural adsorbents are prominent value-added products from agricultural waste materials. Herein, microcrystalline cellulose (MCC) was produced from nipa palm shells (with a purity of 96.2 %), and its application in the metal ion recovery was investigated. The surface morphology and chemical properties of the synthesized MCC were characterized using scanning electron microscopy and spectroscopic methods. While the as-synthesized MCC demonstrated effective metal adsorption capability, it exhibited low selectivity for nickel (II) ion (Ni2+) and cobalt (II) ion (Co2+). To overcome this limitation, we suggest the simultaneous use of the synthesized MCC as an adsorbent for lithium ion (Li+) and a n-butylamine phosphinate ionic liquid [C4H9NH3][Cyanex 272] as an organic extractant for the efficient recovery of Ni2+ and Co2+ contained in spent lithium-ion batteries. Various parameters affecting the efficiency of the extraction process were optimized, including pH, reagent ratios and extraction time. The results revealed that using both MCC and [C4H9NH3][Cyanex 272] ionic liquid considerably improved the metal extraction efficiency, especially for Co2+. The Ni2+ and Co2+ separation efficiency by the ionic liquid was found to be higher than 90 %, highlighting the potential of this combined strategy for recycling valuable metals from spent batteries.
从废lib中回收关键金属的绿色高效双策略:Nipa棕榈壳衍生纤维素和[C4H9NH3][Cyanex 272]离子液体萃取
绿色环保的天然吸附剂是农业废弃物中突出的高附加值产品。以尼帕棕榈壳为原料制备了纯度为96.2%的微晶纤维素(MCC),并对其在金属离子回收中的应用进行了研究。利用扫描电镜和光谱学方法对合成的MCC的表面形貌和化学性质进行了表征。合成的MCC具有较好的金属吸附能力,但对镍(II)离子(Ni2+)和钴(II)离子(Co2+)的选择性较低。为了克服这一限制,我们建议同时使用合成的MCC作为锂离子(Li+)的吸附剂和磷酸正丁胺离子液体[C4H9NH3][Cyanex 272]作为有机萃取剂,以有效回收废锂离子电池中含有的Ni2+和Co2+。对pH、配比、萃取时间等影响萃取效率的参数进行了优化。结果表明,使用MCC和[C4H9NH3][Cyanex 272]离子液体均可显著提高金属萃取效率,尤其是对Co2+的萃取效率。离子液体对Ni2+和Co2+的分离效率高于90%,突出了这种组合策略在回收废电池中有价值金属方面的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


