Eco-friendly microextraction technique using SFO-DLLME coupled with HPTLC for plasma analysis of metformin and imeglimin
IF 2.8
3区 医学
Q2 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
Abstract
This study aimed to develop and validate a green, sensitive, and high-throughput analytical method for the simultaneous determination of metformin and imeglimin in plasma samples using gliclazide as an internal standard. A novel microextraction technique was proposed, combining salt-induced dispersive liquid–liquid microextraction based on solidified floating organic droplet (SI-DLLME-SFOD) with high-performance thin-layer chromatography HPTLC-densitometric detection. This approach significantly improves greenness, sensitivity, and simplicity compared to conventional extraction and chromatographic techniques. The method was optimized for maximum resolution using silica gel 60 F254 pre-coated TLC plates as the stationary phase and a mobile phase comprising methanol: chloroform: 0.5 % w/v ammonium acetate (8:1:1, v/v/v). Detection was performed at 254 nm. The method was validated following US FDA bioanalytical guidelines. The Rf values were observed at 0.45 ± 0.04 for imeglimin, 0.63 ± 0.04 for metformin, and 0.84 ± 0.04 for gliclazide. The method exhibited excellent linearity in the range of 100–1000 ng/band, with correlation coefficients (r2) exceeding 0.998. The average recoveries were 90.91 % for imeglimin and 92.61 % for metformin, with RSD values of 0.42 and 0.39, respectively, confirming high precision and accuracy. The SI-DLLME-SFOD protocol enables efficient extraction, lower solvent consumption, and improved analyte enrichment. The developed method was evaluated using green analytical chemistry metrics and its eco-friendliness was confirmed. It demonstrated reduced solvent usage, minimal waste generation, and a lower environmental burden, establishing its superiority over previously reported sample preparation techniques. Furthermore, the validated method was successfully applied to real samples (human plasma) to assess the concentration, thereby demonstrating the potential value of the method for routine use. This method provides a green, efficient, and validated strategy for the bioanalysis of antidiabetic drugs in human plasma, supporting its potential application in routine clinical diagnostics and therapeutic drug monitoring.
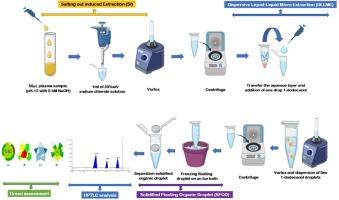
SFO-DLLME结合HPTLC的环保微萃取技术分析血浆中二甲双胍和依米明
本研究旨在建立并验证一种绿色、灵敏、高通量的分析方法,以格列齐特为内标同时测定血浆样品中的二甲双胍和依米明。提出了一种新型的微萃取技术,将基于固化漂浮有机液滴(SI-DLLME-SFOD)的盐诱导分散液-液微萃取与高效薄层色谱- hptlc密度检测相结合。与传统的提取和色谱技术相比,该方法显着提高了绿色,灵敏度和简单性。以硅胶60f254预包覆TLC板为固定相,以甲醇:氯仿:0.5% w/v乙酸铵(8:1:1,v/v/v)为流动相,优化了该方法的最大分辨率。在254 nm处检测。该方法按照美国FDA生物分析指南进行了验证。依美霉素的Rf值为0.45±0.04,二甲双胍为0.63±0.04,格列齐特为0.84±0.04。在100 ~ 1000 ng/波段范围内线性良好,相关系数(r2)大于0.998。伊美霉素和二甲双胍的平均加样回收率分别为90.91%和92.61%,RSD值分别为0.42和0.39,具有较高的精密度和准确度。SI-DLLME-SFOD协议可实现高效萃取,降低溶剂消耗,并改善分析物富集。用绿色分析化学指标对该方法进行了评价,证实了其生态友好性。它证明了溶剂使用量减少,废物产生最少,环境负担较低,与以前报道的样品制备技术相比,确立了其优越性。此外,验证的方法成功地应用于实际样品(人血浆)来评估浓度,从而证明了该方法在日常使用中的潜在价值。该方法为人体血浆中抗糖尿病药物的生物分析提供了一种绿色、高效、有效的方法,支持其在常规临床诊断和治疗药物监测中的潜在应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Chromatography B
医学-分析化学
CiteScore
5.60
自引率
3.30%
发文量
306
审稿时长
44 days
期刊介绍:
The Journal of Chromatography B publishes papers on developments in separation science relevant to biology and biomedical research including both fundamental advances and applications. Analytical techniques which may be considered include the various facets of chromatography, electrophoresis and related methods, affinity and immunoaffinity-based methodologies, hyphenated and other multi-dimensional techniques, and microanalytical approaches. The journal also considers articles reporting developments in sample preparation, detection techniques including mass spectrometry, and data handling and analysis.
Developments related to preparative separations for the isolation and purification of components of biological systems may be published, including chromatographic and electrophoretic methods, affinity separations, field flow fractionation and other preparative approaches.
Applications to the analysis of biological systems and samples will be considered when the analytical science contains a significant element of novelty, e.g. a new approach to the separation of a compound, novel combination of analytical techniques, or significantly improved analytical performance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


