Exceptionally Potent Chiral Anandamide Analogs
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
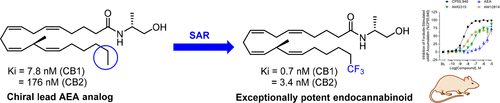
We recently reported an innovative design approach that allowed us to obtain potent endocannabinoids with enhanced metabolic stability. Our design is characterized by the incorporation of chiral centers within the endocannabinoid prototypes N-arachidonoylethanolamide and 2-arachidonoylglycerol. Work on N-arachidonoylethanolamide led to the identification of the first-generation lead analog (R)-N-(1-Methyl-2-hydroxyethyl)-13-(S)-methyl-arachidonamide (AMG315). Here, we synthesized a series of tail-modified AMG315 analogs to further optimize this novel chemotype for cannabinoid receptor binding affinity and potency. Our advanced molecule, namely, 20,20,20-trifluoro-(R)-N-(1-methyl-2-hydroxyethyl)-13-(S)-methyl-arachidonamide (AM12814, 12), is the first endocannabinoid analogue exhibiting unprecedented affinity for both the CB1 and CB2 receptors. In further in vitro functional characterization, 12 behaves as a potent, partial CB1 and CB2 agonist. Our SAR results are supported by docking studies of 12 on the crystal structures of cannabinoid receptors, while when tested in vivo, 12 behaves as a very potent and efficacious CB1 agonist. This analogue will serve as a unique endocannabinoid probe.
特别有效的手性阿南达胺类似物
我们最近报道了一种创新的设计方法,使我们能够获得具有增强代谢稳定性的强效内源性大麻素。我们的设计的特点是在内源性大麻素原型n -花生四烯醇乙醇酰胺和2-花生四烯醇甘油中加入了手性中心。对n -花生四烯酰基乙醇酰胺的研究鉴定出第一代铅类似物(R)- n -(1-甲基-2-羟乙基)-13-(S)-甲基-花生四烯酰胺(AMG315)。在这里,我们合成了一系列尾巴修饰的AMG315类似物,以进一步优化这种新型化学型的大麻素受体结合亲和力和效力。我们的先进分子,即20,20,20-三氟-(R)- n -(1-甲基-2-羟乙基)-13-(S)-甲基-花生四烯酰胺(am12814,12),是第一个内源大麻素类似物,对CB1和CB2受体都具有前所未有的亲和力。在进一步的体外功能表征中,12表现为有效的部分CB1和CB2激动剂。我们的SAR结果得到了12对大麻素受体晶体结构的对接研究的支持,而在体内测试时,12表现为一种非常有效的CB1激动剂。这种类似物将作为一种独特的内源性大麻素探针。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


