Mining the CD4 antigen repertoire for next-generation tuberculosis vaccines
IF 42.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Tuberculosis (TB) is the leading cause of death from infectious disease worldwide, and Bacillus Calmette-Guérin (BCG) remains the only clinically approved vaccine. An enduring challenge in TB vaccine development is systematic antigen selection from a large repertoire of potential candidates. We performed an efficacy screen in mice of antigens that are targets of CD4 T cells in humans. We found striking heterogeneity in protective efficacy, and most of the top protective antigens are not currently in clinical development. We observed immunologic cross-reactivity among phylogenetically clustered antigens, reflecting common CD4 epitopes. We developed a trivalent mRNA vaccine consisting of PPE20 (Rv1387), EsxG (Rv0287), and PE18 (Rv1788), which augmented and exceeded BCG protection in multiple mouse models. Finally, we observed cellular immune responses to these antigens in 84% of humans exposed to M. tuberculosis. These data advance our understanding of TB vaccine immunology and define a vaccine concept for clinical development.
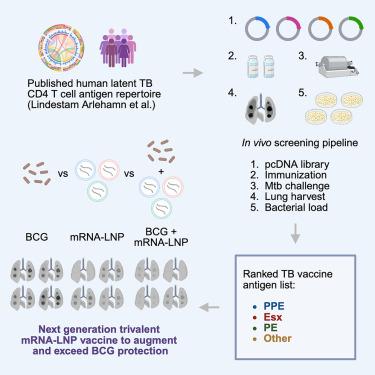
为下一代结核病疫苗挖掘CD4抗原库
结核病(TB)是全世界传染病死亡的主要原因,卡介苗(BCG)仍然是唯一临床批准的疫苗。结核病疫苗开发的一个长期挑战是从大量潜在候选抗原中系统地选择抗原。我们对人类CD4 T细胞的靶标抗原在小鼠中进行了功效筛选。我们发现在保护效果上存在显著的异质性,并且大多数顶级保护性抗原目前尚未进入临床开发阶段。我们观察到系统发育聚集抗原之间的免疫交叉反应性,反映了共同的CD4表位。我们开发了一种由PPE20 (Rv1387)、EsxG (Rv0287)和PE18 (Rv1788)组成的三价mRNA疫苗,在多种小鼠模型中增强并超过了BCG的保护作用。最后,我们观察到84%暴露于结核分枝杆菌的人对这些抗原的细胞免疫反应。这些数据促进了我们对结核病疫苗免疫学的理解,并为临床开发确定了疫苗概念。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell
生物-生化与分子生物学
CiteScore
110.00
自引率
0.80%
发文量
396
审稿时长
2 months
期刊介绍:
Cells is an international, peer-reviewed, open access journal that focuses on cell biology, molecular biology, and biophysics. It is affiliated with several societies, including the Spanish Society for Biochemistry and Molecular Biology (SEBBM), Nordic Autophagy Society (NAS), Spanish Society of Hematology and Hemotherapy (SEHH), and Society for Regenerative Medicine (Russian Federation) (RPO).
The journal publishes research findings of significant importance in various areas of experimental biology, such as cell biology, molecular biology, neuroscience, immunology, virology, microbiology, cancer, human genetics, systems biology, signaling, and disease mechanisms and therapeutics. The primary criterion for considering papers is whether the results contribute to significant conceptual advances or raise thought-provoking questions and hypotheses related to interesting and important biological inquiries.
In addition to primary research articles presented in four formats, Cells also features review and opinion articles in its "leading edge" section, discussing recent research advancements and topics of interest to its wide readership.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


