A spatial map of MAPK-activated immunosuppressive myeloid populations in pediatric low-grade glioma
IF 27.6
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
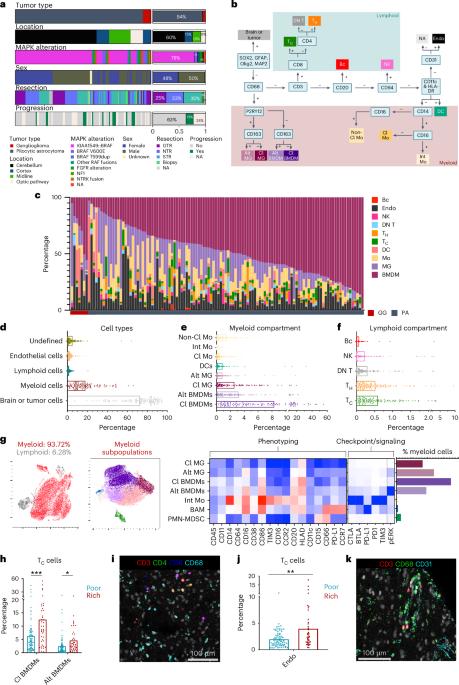
Pediatric low-grade gliomas (pLGGs) are mitogen-activated protein kinase (MAPK) pathway-activated brain tumors prevalent in children and are associated with morbidity despite favorable survival. Here using imaging mass cytometry, we spatially characterized at the single-cell level the tumor microenvironment (TME) of 120 pLGG cases, considering age, molecular drivers, brain location and tumor subtype. Our analysis identified myeloid cells—including resident microglia and bone marrow-derived macrophages—as the predominant immune population in the TME, particularly in optic pathway tumors. Additionally, we discovered an immune signature predictive of progression-free survival. Spatial analysis identified specific cellular interactions, notably myeloid–myeloid contacts and macrophage-enriched regions harboring MAPK-activated, TIM-3+ myeloid cells, suggesting an immunosuppressive TME. Our study provides a comprehensive resource on the immune landscape of these pLGGs and underscores the immunosuppressive role of diverse myeloid infiltrates. These findings also indicate that combining TIM-3 blockade with MAPK inhibition might be a promising therapeutic strategy to target both the TME and oncogenic MAPK activation in pLGG tumors. Here the authors spatially map human pediatric low-grade gliomas using imaging mass cytometry, focusing on immunosuppressive myeloid cell populations and their functionality in tumor–immune interactions and tumor progression.
儿童低级别胶质瘤中mapk激活的免疫抑制髓细胞群的空间图
小儿低级别胶质瘤(pLGGs)是一种在儿童中普遍存在的丝裂原活化蛋白激酶(MAPK)途径激活的脑肿瘤,尽管生存率较高,但其发病率较高。在这里,我们使用成像细胞术在单细胞水平上对120例pLGG病例的肿瘤微环境(TME)进行了空间表征,考虑了年龄、分子驱动因素、脑位置和肿瘤亚型。我们的分析确定髓系细胞——包括常驻小胶质细胞和骨髓来源的巨噬细胞——是TME中主要的免疫群体,特别是在视神经通路肿瘤中。此外,我们还发现了一种预测无进展生存期的免疫特征。空间分析发现了特定的细胞相互作用,特别是髓-髓细胞接触和巨噬细胞富集区域,这些区域含有mapk激活的TIM-3+髓细胞,表明TME具有免疫抑制作用。我们的研究为这些pLGGs的免疫景观提供了一个全面的资源,并强调了不同骨髓浸润的免疫抑制作用。这些发现还表明,结合TIM-3阻断和MAPK抑制可能是一种有希望的治疗策略,可以同时针对pLGG肿瘤的TME和致癌MAPK激活。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


