The CPK3-mediated cytosolic–nuclear translocation of bHLH107 recruits HY5 to regulate the Cu2+-triggered upregulation of ACS8
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Introduction
Copper serves as a key component of plant micronutrients and copper-based antimicrobial compounds (CBACs), and it has been used to protect plants against diseases for more than 130 years. We previously revealed that low concentrations of Cu2+ elicit plant immune responses by activating the expression of the ethylene synthesis rate-limiting enzyme 1-aminocyclopropanecarboxylic acid (ACC) synthesis 8 (ACS8), which depends on the copper response element (CuRE) in the promoter. However, the transcription regulatory mechanism of Cu2+-triggered immunity upstream of ACS8 remains unclear.Objectives
Here, we aimed to identify the CuRE-binding transcription factor (CuTF) and elucidate it roles in activating the Cu2+-induced expression of ACS8.Methods
To identify the CuTF and its interactors, we performed DNA-pull-down and mass spectrometry and protein phosphorylation proteomics assays. Cu2+-induced plant immune responses were conducted assays measuring the ACS8 expression, bacterial populations and RNA-seq. Additionally, protein-DNA and protein–protein interactions were performed using yeast one hybridization, electrophoretic mobility shift assay (EMSA), chromatin immunoprecipitation-quantitative polymerase chain reaction (ChIP-qPCR), pull-down, luciferase complementation assay, and coimmunopreciptitation assay.Results
We identified a CuTF of basic helix-loop-helix 107 (bHLH107) which is required for Cu2+-triggered activation of ACS8 expression and resistance to Pseudomonas syringae pv. tomato (Pst) DC3000. Calcium-dependent protein kinase 3 (CPK3) interacts with and phosphorylates bHLH107 at Ser62 and Ser72 to mediate bHLH107 translocation from the cytoplasm into the nucleus, leading to an increase in the transactivation activity of bHLH107. Moreover, our findings revealed that bHLH107 interacts with Arabidopsis ELONGATED HYPOCOTYL5 (HY5) in nucleus. HY5 directly binds to the ACGT-containing elements (ACE-box) and acts as a coactivator to promote bHLH107 binding to the CuRE cis-element and to increase transcription of ACS8 upon Cu2+ treatment.Conclusion
Overall, we revealed a CPK3-bHLH107-HY5 module that regulates the network of Cu2+-triggered plant immunity upstream of ACS8 that is involved in the cytosolic-nuclear translocation of bHLH107.
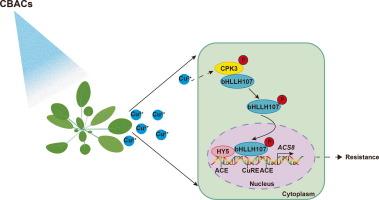
cpk3介导的bHLH107细胞质核易位招募HY5来调节Cu2+引发的ACS8上调
铜作为植物微量营养素和铜基抗微生物化合物(CBACs)的关键成分,已被用于保护植物免受疾病侵袭130 多年。我们之前发现,低浓度的Cu2+通过激活乙烯合成限速酶1-氨基环丙烷羧酸(ACC)合成8 (ACS8)的表达引发植物免疫应答,这取决于启动子中的铜反应元件(CuRE)。然而,ACS8上游Cu2+触发免疫的转录调控机制尚不清楚。目的研究Cu2+诱导ACS8表达的Cu2+结合转录因子(CuRE-binding transcription factor, cuf)。方法采用DNA-pull-down、质谱分析和蛋白磷酸化蛋白质组学方法鉴定该蛋白及其相互作用物。对Cu2+诱导的植物免疫应答进行测定,测定ACS8的表达、细菌数量和RNA-seq。此外,蛋白质- dna和蛋白质-蛋白质相互作用通过酵母杂交、电泳迁移迁移试验(EMSA)、染色质免疫沉淀-定量聚合酶链反应(ChIP-qPCR)、拉下、荧光素酶互补试验和共免疫沉淀试验进行。结果鉴定出Cu2+激活ACS8表达并对丁香假单胞菌产生抗性所需的碱性螺旋-环-螺旋107 (bHLH107)切蛋白。番茄(Pst) DC3000。钙依赖性蛋白激酶3 (Calcium-dependent protein kinase 3, CPK3)与bHLH107相互作用,使bHLH107在Ser62和Ser72位点磷酸化,介导bHLH107从细胞质转位到细胞核,导致bHLH107的转激活活性增加。此外,我们的研究结果表明bHLH107与拟南芥细胞核中的伸长HYPOCOTYL5 (HY5)相互作用。HY5直接结合ACGT-containing elements (ACE-box),作为辅激活因子促进bHLH107与CuRE顺式元件结合,并在Cu2+处理下增加ACS8的转录。总之,我们发现了一个CPK3-bHLH107-HY5模块,该模块调节ACS8上游Cu2+触发的植物免疫网络,参与bHLH107的细胞质-核易位。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


