Novel Substitutes of Phthalate Esters (PAEs) Promote the Propagation of Antibiotic Resistance Genes via Ferroptosis: Implication for the Environmental Safety Evaluation of PAE Substitutes
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
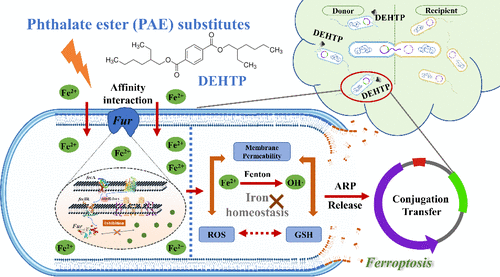
The horizontal transfer of antibiotic resistance genes (ARGs) has become a major threat to global public health. Recent studies have found that ferroptosis, an iron-dependent form of regulated cell death characterized by lipid peroxidation and glutathione depletion, may play a critical role in the dissemination of ARGs among environmental microbes. Here, we demonstrated for the first time that phthalate esters (PAEs) and their substitutes significantly enhanced plasmid conjugation by triggering ferroptosis-related pathways. Classical ferroptosis-associated responses, including the hyperpolarization of the cell membrane potential, elevated production of reactive oxygen species, and heightened membrane permeability, were observed under the stress of PAEs or their substitutes. Through integrated transcriptomic and metabolomic analyses, we revealed that these compounds triggered iron dysregulation via the upregulation of iron acquisition and storage pathways while suppressing DNA replication, concurrently causing oxidative damage that stimulated the plasmid conjugation. Molecular docking simulations revealed that PAEs and their substitutes competitively disrupted the functionality of ferric uptake regulator (Fur) protein, a master controller of intracellular iron homeostasis, with superior binding affinity than its natural ligand Fe2+. Integrated metagenomic sequencing and homology analyses demonstrated the conservation of Fur protein across biofilm microbiota and functional implications in iron homeostasis. Structural analysis based on the characteristic molecular fingerprints of chemicals pinpointed aliphatic chains as the crucial structure responsible for enhancing ARG propagation between bacteria. Our findings uncovered a mechanism by which PAEs and their substitutes exacerbated ARG dissemination through ferroptosis-mediated conjugation, providing crucial insights for environmental risk assessment and resistance mitigation strategies.
新型邻苯二甲酸酯(PAEs)替代物通过嗜铁作用促进抗生素耐药基因的繁殖:对PAE替代物环境安全性评价的意义
抗生素耐药基因(ARGs)的水平转移已成为全球公共卫生的主要威胁。最近的研究发现,铁死亡是一种以脂质过氧化和谷胱甘肽耗竭为特征的铁依赖性细胞死亡形式,可能在环境微生物中ARGs的传播中起关键作用。在这里,我们首次证明邻苯二甲酸酯(PAEs)及其替代品通过触发铁凋亡相关途径显着增强质粒结合。在PAEs或其替代品的胁迫下,观察到典型的死铁相关反应,包括细胞膜电位的超极化、活性氧的增加和膜通透性的提高。通过综合转录组学和代谢组学分析,我们发现这些化合物通过上调铁获取和储存途径引发铁调节失调,同时抑制DNA复制,同时引起氧化损伤,刺激质粒结合。分子对接模拟显示,PAEs及其替代品竞争性地破坏了铁摄取调节蛋白(Fur)的功能,该蛋白是细胞内铁稳态的主要控制因子,其结合亲和力优于其天然配体Fe2+。综合宏基因组测序和同源性分析证明了Fur蛋白在生物膜微生物群中的保守性及其在铁稳态中的功能意义。基于化学物质特征分子指纹的结构分析确定脂肪链是促进细菌间ARG繁殖的关键结构。我们的研究结果揭示了PAEs及其替代品通过嗜铁细胞介导的偶联加剧ARG传播的机制,为环境风险评估和耐药性缓解策略提供了重要见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

环境科学与技术
环境科学-工程:环境
CiteScore
17.50
自引率
9.60%
发文量
12359
审稿时长
2.8 months
期刊介绍:
Environmental Science & Technology (ES&T) is a co-sponsored academic and technical magazine by the Hubei Provincial Environmental Protection Bureau and the Hubei Provincial Academy of Environmental Sciences.
Environmental Science & Technology (ES&T) holds the status of Chinese core journals, scientific papers source journals of China, Chinese Science Citation Database source journals, and Chinese Academic Journal Comprehensive Evaluation Database source journals. This publication focuses on the academic field of environmental protection, featuring articles related to environmental protection and technical advancements.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


