Cafeteria diet increases approach behavior and appetitive ultrasonic vocalizations triggered by a food-related cue in male rats
IF 2.5
3区 医学
Q2 BEHAVIORAL SCIENCES
引用次数: 0
Abstract
The omnipresence of highly palatable food and its related cues constitutes an important risk factor for overeating and body weight gain in today's obesogenic environment. This study examined the metabolic, behavioral, and neurobiological effects of a cafeteria (CAF) diet (kcal composition: 42 % carbs, 13 % protein, 45 % fat) against two control grain-based diets: C1 (kcal composition: 63 % carbs, 23 % protein, 14 % fat) and C2 (kcal composition: 58 % carbs, 29 % protein, 13 % fat) in male Wistar rats (n = 27) at postnatal day 38. After a 9-week feeding period, open-field activity and ultrasonic vocalizations (USVs) were assessed using the animals' empty food container to evaluate attribution of incentive salience to food cues. Additionally, we measured biochemical serum profiles, neurotransmitter levels, and mRNA for BDNF, TrkB, CREB, Dnmt3A, and CRF in reward-related brain regions. Results showed that the CAF diet increased food intake, body weight, and adiposity. CAF-fed rats significantly explored the empty food container more and emitted higher rates of 50-kHz frequency-modulated USVs –markers of incentive motivation and positive affect. The CAF diet also upregulated hippocampal BDNF, TrkB, and CREB, while downregulated TrkB, CREB, and Dnmt3A mRNA in the nucleus accumbens. Although both control diets were suitable for studying CAF effects, the C1 and C2 groups differed in some parameters (e.g., mRNA, cholesterol, and glutamate levels), highlighting the need for appropriate control diets. Our findings reveal that the CAF diet enhances behavioral reactivity to food cues and induces distinct neurobiological alterations, shedding light on the mechanisms linking palatable foods, reward processing, and obesity vulnerability.
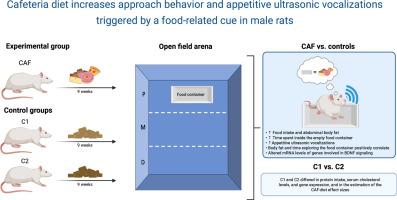
自助餐厅饮食增加了雄性大鼠的接近行为和由食物相关线索引发的食欲超声发声。
在当今的致肥环境中,无处不在的美味食物及其相关线索构成了暴饮暴食和体重增加的重要风险因素。本研究在雄性Wistar大鼠(n=27)出生后第38天,检测了自助餐厅(CAF)饮食(卡路里组成:42%碳水化合物,13%蛋白质,45%脂肪)与两种对照谷物饮食(C1(卡路里组成:63%碳水化合物,23%蛋白质,14%脂肪)和C2(卡路里组成:58%碳水化合物,29%蛋白质,13%脂肪)的代谢,行为和神经生物学效应。在9周的喂养期后,利用动物的空食物容器评估开放场地活动和超声波发声(usv),以评估食物线索对激励显著性的归因。此外,我们测量了生化血清谱、神经递质水平和BDNF、TrkB、CREB、Dnmt3A和CRF在奖励相关脑区的mRNA水平。结果表明,CAF饮食增加了食物摄入量、体重和肥胖。咖啡因喂养的大鼠更多地探索空的食物容器,并发出更高的50 khz调频usv -激励动机和积极情绪的标志。CAF饮食也上调海马BDNF、TrkB和CREB,下调伏隔核TrkB、CREB和Dnmt3A mRNA。虽然两种对照饲料都适合研究CAF效应,但C1组和C2组在一些参数(如mRNA、胆固醇和谷氨酸水平)上存在差异,这突出表明需要适当的对照饲料。我们的研究结果表明,CAF饮食增强了对食物线索的行为反应,并诱导了明显的神经生物学改变,揭示了美味食物、奖励处理和肥胖易感性之间的机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Physiology & Behavior
医学-行为科学
CiteScore
5.70
自引率
3.40%
发文量
274
审稿时长
47 days
期刊介绍:
Physiology & Behavior is aimed at the causal physiological mechanisms of behavior and its modulation by environmental factors. The journal invites original reports in the broad area of behavioral and cognitive neuroscience, in which at least one variable is physiological and the primary emphasis and theoretical context are behavioral. The range of subjects includes behavioral neuroendocrinology, psychoneuroimmunology, learning and memory, ingestion, social behavior, and studies related to the mechanisms of psychopathology. Contemporary reviews and theoretical articles are welcomed and the Editors invite such proposals from interested authors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


