Time shift in ciclosporin metabolism downregulation by inflammation in allogeneic hematopoietic cell transplantation: A time-dependent transduction modelling PK-PD approach
IF 4.7
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Ciclosporin is a major immunosuppressant in allogeneic hematopoietic stem cell transplantation (HSCT), with a narrow therapeutic index and high interindividual variability. We previously showed that severe inflammation significantly reduces cytochrome P450 3A4 (CYP3A4)-mediated ciclosporin metabolism, as reflected by an increased concentration-to-dose (C/D) ratio. However, the temporal dynamics between inflammation and ciclosporin metabolism remain poorly understood. This study aimed to characterize the time-dependent effect of inflammation—quantified by C-reactive protein (CRP) levels—on CYP3A4-mediated ciclosporin metabolism using a signal transduction pharmacokinetic-pharmacodynamic (PK-PD) modeling approach in HSCT patients. We selected 10 HSCT patients with a single moderate-to-severe inflammatory episode (CRP > 40 mg/L) and modeled CRP kinetics alongside ciclosporin C/D ratios using a transduction model. Key parameters included the maximum effect (Emax), the CRP concentration at 50% of Emax (EC50), and the time delay (τ) between CRP and metabolism variations. External validation was performed using published time profiles from Chen et al. 1994 and 2 patients with potential drug–drug interactions (pDDI) involving letermovir from our cohort. The model captured a 4.7-fold increase in C/D ratio at an EC50 of 120 mg/L of CRP, with a time delay of 6 days between CRP peak and C/D ratio peak. External validation confirmed strong predictive performance. In patients with moderate-to-severe inflammation, inflammation-driven CYP3A4 downregulation appeared to outweigh the impact of letermovir-mediated DDI. Our results provide the first quantitative evidence of time-dependent inhibition due to inflammation of ciclosporin metabolism. This quantitative modeling framework may inform dose adjustment and DDI risk evaluation in inflammatory conditions, particularly in allogeneic HSCT patients.
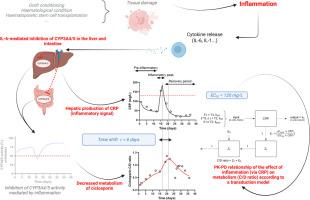
异基因造血细胞移植中炎症引起环孢素代谢下调的时移:一种时间依赖性转导模型PK-PD方法。
环孢素是异基因造血干细胞移植(HSCT)的主要免疫抑制剂,治疗指数窄,个体间差异大。我们之前的研究表明,严重的炎症会显著降低细胞色素P450 3A4 (CYP3A4)介导的环孢素代谢,这反映在浓度剂量比(C/D)的增加上。然而,炎症和环孢素代谢之间的时间动力学仍然知之甚少。本研究旨在通过信号转导药代动力学-药效学(pkpd)建模方法,表征炎症(通过c反应蛋白(CRP)水平量化)对HSCT患者cyp3a4介导的环孢素代谢的时间依赖性影响。我们选择了10例单一中度至重度炎症发作的HSCT患者(CRP > 40 mg/L),并使用转导模型模拟CRP动力学和环孢素C/D比率。关键参数包括最大效应(Emax)、50% Emax时CRP浓度(EC50)和CRP与代谢变化之间的延迟时间(τ)。外部验证采用Chen等人1994年发表的时间档案和我们队列中2例涉及利特莫韦的潜在药物-药物相互作用(pDDI)患者进行。在EC50浓度为120 mg/L时,模型捕获到C/D比增加4.7倍,CRP峰值与C/D比峰值之间的时间延迟6天。外部验证证实了强大的预测性能。在中度至重度炎症患者中,炎症驱动的CYP3A4下调似乎超过了莱特莫韦介导的DDI的影响。我们的结果提供了环孢素代谢炎症引起的时间依赖性抑制的第一个定量证据。这种定量建模框架可以为炎症条件下的剂量调整和DDI风险评估提供信息,特别是在异体造血干细胞移植患者中。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


