Supersaturation of self-nanoemulsifying drug delivery systems: Correlation between equilibrium solubility and supersaturation concentration, effect on droplet size and impact of polymer addition
IF 4.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
European Journal of Pharmaceutics and Biopharmaceutics
Pub Date : 2025-09-11
DOI:10.1016/j.ejpb.2025.114862
引用次数: 0
Abstract
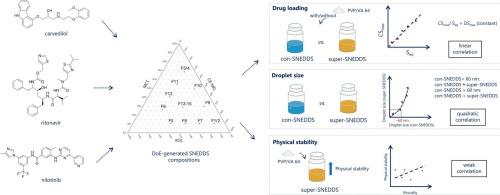
Supersaturated self-nanoemulsifying drug delivery systems (super-SNEDDS) have emerged as a promising approach to increase the drug load of hydrophobic molecules, compared to conventional SNEDDS (con-SNEDDS). This study aimed to explore the relationship between equilibrium solubility (Seq) and the maximum supersaturation concentration (CSmax) in SNEDDS preconcentrates for three model drugs: carvedilol (CVL), ritonavir (RTV) and nilotinib (NTB). The emulsion droplet size of con-SNEDDS (90 % of Seq) and super-SNEDDS (90 % of CSmax), and the influence of polyvinylpyrrolidone-vinyl acetate copolymers 64 (PVP/VA 64) was assessed, as well as the relationship between physical stability and viscosity of super-SNEDDS in the presence of PVP/VA 64. A Design of Experiment (DoE) approach was applied to optimize SNEDDS compositions. The results showed linear correlations between Seq and CSmax across all three model drugs, leading to a consistent (but drug dependent) maximum degree of supersaturation (DSmax) (CVL = 2.49, RTV = 4.56, NTB = 2.54) within the DoE design space. Dissolving 4 % (w/w) PVP/VA 64 in the SNEDDS preconcentrates did not influence the described correlations or DSmax. Emulsion droplet size remained unchanged upon drug loading to 90 % of Seq (con-SNEDDS) compared to drug-free SNEDDS. Further loading to 90 % of CSmax (super-SNEDDS) also resulted in negligible size changes in emulsions with initial droplet sizes below 60 nm, whereas those above 60 nm exhibited pronounced droplet size increase, following a quadratic relationship compared with their initial con-SNEDDS droplet size. Incorporation of PVP/VA 64 enhanced both the physical stability and viscosity of super-SNEDDS; however, only a weak correlation was observed between these two parameters, suggesting that viscosity alone did not govern the stabilization of super-SNEDDS. In summary, within the design space, DSmax is drug-dependent, but independent of SNEDDS composition or polymer addition; droplet size of super-SNEDDS is dependent on both drug and SNEDDS composition, but is unaffected by polymer; in contrast, physical stability is jointly influenced by drug properties, SNEDDS composition, and polymer addition.
自纳米乳化给药系统的过饱和:平衡溶解度与过饱和浓度的关系,对液滴大小的影响以及聚合物添加的影响。
与传统的SNEDDS (con-SNEDDS)相比,过饱和自纳米乳化药物递送系统(super-SNEDDS)是一种很有前途的方法,可以增加疏水分子的药物负荷。本研究旨在探讨三种模型药物卡维地洛(CVL)、利托那韦(RTV)和尼罗替尼(NTB)在SNEDDS预浓缩物中平衡溶解度(Seq)与最大过饱和浓度(CSmax)的关系。考察了con-SNEDDS(占Seq的90 %)和super-SNEDDS(占CSmax的90 %)的乳液滴度、聚乙烯吡咯烷酮-醋酸乙烯共聚物64 (PVP/VA 64)对其性能的影响,以及PVP/VA 64存在下super-SNEDDS物理稳定性与粘度的关系。采用实验设计法(Design of Experiment, DoE)对SNEDDS的组成进行优化。结果显示,在所有三种模型药物中,Seq和CSmax之间存在线性相关性,导致DoE设计空间内最大过饱和度(DSmax)一致(但依赖于药物)(CVL = 2.49, RTV = 4.56, NTB = 2.54)。在SNEDDS预浓缩物中溶解4 % (w/w) PVP/VA 64不影响上述相关性或DSmax。与无药SNEDDS相比,载药后乳滴大小保持不变,达到Seq的90% % (con-SNEDDS)。当CSmax (super-SNEDDS)添加量达到90% %时,初始液滴尺寸小于60 nm的乳剂的尺寸变化可以忽略不计,而当CSmax (super-SNEDDS)添加量大于60 nm时,乳剂的尺寸与初始con-SNEDDS的液滴尺寸相比呈二次曲线关系明显增大。PVP/VA 64的掺入提高了super-SNEDDS的物理稳定性和粘度;然而,这两个参数之间只有微弱的相关性,这表明粘度本身并不能控制超级snedds的稳定性。综上所述,在设计空间内,DSmax依赖于药物,但不依赖于SNEDDS组合物或聚合物添加;超SNEDDS的液滴大小取决于药物和SNEDDS的组成,但不受聚合物的影响;相反,物理稳定性受药物性质、SNEDDS组成和聚合物加成的共同影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.80
自引率
4.10%
发文量
211
审稿时长
36 days
期刊介绍:
The European Journal of Pharmaceutics and Biopharmaceutics provides a medium for the publication of novel, innovative and hypothesis-driven research from the areas of Pharmaceutics and Biopharmaceutics.
Topics covered include for example:
Design and development of drug delivery systems for pharmaceuticals and biopharmaceuticals (small molecules, proteins, nucleic acids)
Aspects of manufacturing process design
Biomedical aspects of drug product design
Strategies and formulations for controlled drug transport across biological barriers
Physicochemical aspects of drug product development
Novel excipients for drug product design
Drug delivery and controlled release systems for systemic and local applications
Nanomaterials for therapeutic and diagnostic purposes
Advanced therapy medicinal products
Medical devices supporting a distinct pharmacological effect.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


