Identification and characterization of a DNA-binding protein from starved cells (Dps) homologue in Acanthamoeba: Implications for encystment-induced DNA protection
IF 2.5
3区 医学
Q2 PARASITOLOGY
引用次数: 0
Abstract
Acanthamoeba spp. are free-living amoebae recognized as opportunistic human pathogens. Under harsh conditions, trophozoites transform into cysts, undergoing degradation of internal structures and macromolecules, including DNA, which enhances their survival by increasing resistance. However, the mechanism underlying DNA protection in protozoa, such as Acanthamoeba, during the encystment under adverse conditions remains unclear. We cloned a bacterial Dps homologue (AcDps) from Acanthamoeba, then expressed and purified the recombinant AcDps to investigate its functional characteristics. Expression of AcDps is minimal in trophozoites but initiates following encystment induction. When tagged with EGFP, AcDps appears as multiple small vesicle-like structures scattered throughout the cysts' cytoplasm. We examined the factors influencing the formation of rAcDps oligomeric complexes and DNA-rAcDps complexes under various divalent cation and pH conditions by utilizing techniques such as electrophoretic mobility shift assay, and atomic force microscopy imaging. These complexes effectively bind both linear and supercoiled plasmid DNA in the presence of Zn²⁺ divalent cations, creating quasi-DNA complexes that protect the DNA from DNase I degradation. The capacity of rAcDps to oligomerize and bind to DNA, presumed to serve as a protective barrier against damage, highlights its functional and evolutionary importance and suggests its potential as a therapeutic target.
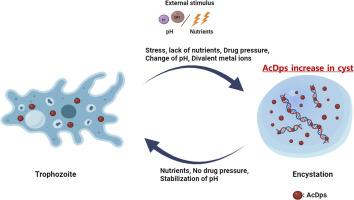
棘阿米巴饥饿细胞(Dps)同源物DNA结合蛋白的鉴定和表征:对系统诱导的DNA保护的意义。
棘阿米巴是一种自由生活的变形虫,被认为是机会性的人类病原体。在恶劣的条件下,滋养体转化为包囊,经历内部结构和大分子(包括DNA)的降解,这通过增加抗性来提高它们的存活率。然而,原生动物(如棘阿米巴)在不利条件下的囊化过程中DNA保护的机制尚不清楚。从棘阿米巴中克隆了一个细菌Dps同源物(AcDps),并对其进行了纯化和表达,研究了其功能特性。AcDps在滋养体中表达最少,但在包囊诱导后开始表达。当被EGFP标记时,AcDps表现为分散在囊肿细胞质中的多个小囊泡样结构。我们利用电泳迁移率转移测定和原子力显微镜成像等技术,研究了在不同二价阳离子和pH条件下影响rAcDps寡聚物和DNA-rAcDps复合物形成的因素。这些复合物在Zn 2 +二价阳离子的存在下有效地结合线性和超螺旋质粒DNA,形成准DNA复合物,保护DNA免受DNA酶I降解。rAcDps寡聚和结合DNA的能力,被认为是防止损伤的保护屏障,突出了其功能和进化的重要性,并表明其作为治疗靶点的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta tropica
医学-寄生虫学
CiteScore
5.40
自引率
11.10%
发文量
383
审稿时长
37 days
期刊介绍:
Acta Tropica, is an international journal on infectious diseases that covers public health sciences and biomedical research with particular emphasis on topics relevant to human and animal health in the tropics and the subtropics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


