Non-thermal atmospheric pressure plasma–liquid synthesis of organic acids in aqueous solution from carbon monoxide
IF 9.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
This work aims at understanding the conversion of CO to organic acids, namely oxalic acid and formic acid, using non-thermal atmospheric pressure plasma over aqueous solutions. CO exhibited significantly higher conversion to organic acids (more than 15×) compared to CO2 under the same reaction conditions. The result bolsters a proposed two-step process for CO2 fixation, whereby CO2 is first converted to CO, and then CO is converted to organic acids. The organic acids produced from CO are intermediates in the water–gas shift (WGS) reaction of CO in the presence of an aqueous solution to dissolved CO2 and hydrogen gas. Based on a simple thermodynamic analysis, the organic acid yield was increased by lowering the plasma–liquid reaction temperature using an ice bath to cool the reaction flask. The composition of the organic acids could be varied by changing the pH of the solution. Oxalate was formed in higher concentrations with increasing solution pH above the pKa of the radical species (CO2)˙−. Below the pKa value, formate was the exclusive organic acid formed. The production of formate has a rather weak pH dependence but is enhanced slightly at a basic pH above 10. Furthermore, at basic pH, the effect of electrolyte concentration comes into play. Higher electrolyte concentrations, leading to shorter electrolyte Debye lengths, resulted in lowered organic acid yields. The highest yields of organic acids obtained in our system were 122 mg L−1 for oxalate and 77 mg L−1 for formate at an optimum 1 mM NaOH concentration in the starting solution. This work is a successful pioneering example of CO to organic acids conversion using non-thermal plasmas, which opens the pathway for a promising two-step conversion process of CO2 to organic acids.
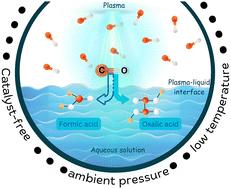
非热常压等离子体-液相合成一氧化碳水溶液中的有机酸
这项工作旨在了解CO转化为有机酸,即草酸和甲酸,使用非热大气压等离子体在水溶液中。在相同的反应条件下,CO对有机酸的转化率明显高于CO2(大于15倍)。这一结果支持了二氧化碳固定的两步过程,即首先将二氧化碳转化为CO,然后将CO转化为有机酸。由CO产生的有机酸是CO在水溶液中与溶解的CO2和氢气发生水气转换(WGS)反应的中间产物。基于简单的热力学分析,采用冰浴冷却反应烧瓶,降低等离子体-液体反应温度,可以提高有机酸的产率。有机酸的组成可以通过改变溶液的pH值而改变。当溶液pH高于自由基(CO2)的pKa时,草酸形成的浓度越高。在pKa值以下,甲酸是唯一形成的有机酸。甲酸酯的生成对pH值的依赖性较弱,但在碱性pH值大于10时,甲酸酯的生成略有增加。此外,在碱性pH下,电解质浓度的影响开始发挥作用。较高的电解质浓度,导致较短的电解质德拜长度,导致降低有机酸产率。在起始溶液中NaOH浓度为1mm时,草酸酯和甲酸酯的有机酸产量最高,分别为122mg L−1和77mg L−1。这项工作是利用非热等离子体将CO转化为有机酸的成功先驱,为有前途的两步二氧化碳转化为有机酸的过程开辟了途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


