Stabilizing ultrahigh-nickel cobalt-free lithium layered oxide cathode via Mg doping: pillar and electromagnetic center roles
IF 5.6
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
Abstract
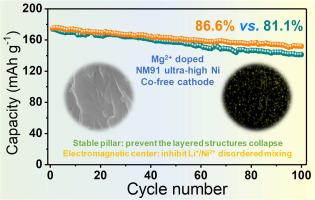
Ultrahigh-nickel cobalt-free lithium layered oxide cathodes are widely researched and applied owing to their high capacity and low cost. Unfortunately, the ultrahigh nickel content implies deep lithium-ion extraction/insertion and intense redox reactions, while the cobalt-free results in poor electronic conductivity and serious Li+/Ni2+ disordering mixing. These factors severely threaten the cycle life and rate capability for battery. In this work, a Mg2+-doped LiNi0.9Mn0.1O2 (NM91 and NM91-Mg) ultrahigh-nickel cobalt-free cathode is prepared to enhance lithium storage performance. Mg2+ doping plays a dual role as both an "electrostatic center" and a "pillar" within the lithium layers. Firstly, Mg2+ possesses more extranuclear electrons than Li+, resulting in stronger electrostatic interactions within the lithium layer. This enables it to act as an electrostatic center, inhibiting disordered cation migration. XRD refinement results confirm that Mg doping suppresses the detrimental Li+/Ni2+ mixing, thereby mitigating the negative effects associated with cobalt-free. Secondly, Mg2+ fixed within the lithium layers acts as a stable pillar species. It helps prevent the layered structure from collapsing during deep lithium-ion extraction. Battery in-situ XRD results show that Mg doping suppresses the harmful H2 to H3 phase transition, alleviating the negative effects caused by the ultrahigh nickel content. Consequently, NM91-Mg demonstrates significantly superior cycle stability (capacity retention for 100 cycles: 86.6 % vs. 81.1 %) and rate capability (discharge capacity at a high rate of 5 C: 98 mAh g-1 vs. 89 mAh g-1) compared to NM91. Remarkably, the constructed full battery also achieves a high capacity retention of 91.7 % after 100 cycles. Mg doping provides targeted improvements for ultrahigh-nickel cobalt-free cathode materials, bringing them closer to practical application.
镁掺杂稳定超高镍无钴锂层状氧化物阴极:支柱和电磁中心作用
超高镍无钴锂层状氧化物阴极以其高容量、低成本的优点得到了广泛的研究和应用。不幸的是,超高的镍含量意味着锂离子的深度萃取/插入和强烈的氧化还原反应,而无钴则导致电子导电性差和严重的Li+/Ni2+无序混合。这些因素严重威胁着电池的循环寿命和倍率性能。本文制备了掺杂Mg2+的LiNi0.9Mn0.1O2 (NM91和NM91- mg)超高镍无钴阴极,以提高锂存储性能。Mg2+掺杂在锂电层中扮演着“静电中心”和“支柱”的双重角色。首先,Mg2+比Li+拥有更多的核外电子,导致锂层内的静电相互作用更强。这使得它作为静电中心,抑制无序阳离子迁移。XRD细化结果证实,Mg掺杂抑制了有害的Li+/Ni2+混合,从而减轻了无钴带来的负面影响。其次,固定在锂层内的Mg2+作为稳定的支柱物质。它有助于防止层状结构在深层锂离子提取过程中坍塌。电池原位XRD结果表明,Mg掺杂抑制了有害的H2到H3的相变,减轻了超高镍含量带来的负面影响。因此,与NM91相比,NM91- mg表现出显著优于NM91的循环稳定性(100次循环的容量保持率:86.6%对81.1%)和倍率能力(5℃高倍率下的放电容量:98 mAh g-1对89 mAh g-1)。值得注意的是,构建的全电池在100次循环后也达到了91.7%的高容量保留率。Mg掺杂为超高镍无钴正极材料提供了有针对性的改进,使其更接近实际应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


