The effects of anionic doping on the ion transport of Li3TMCl6 (TM=Y, Er) and the heterostructure solid electrolytes interface first-principles study
IF 5.6
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
Abstract
Li3YCl6 and Li3ErCl6 are widely used as solid electrolytes in all-solid-state batteries due to their good ionic conductivity and wide stable chemical window. Li3YCl6 and Li3ErCl6, which have a tripartite lattice structure, not only have a high activation energy for transporting ions due to the strong electronegativity of the anion but also have poor interfacial stability with the cathode. The effects of anion X (X = F, Br, and I) doping on the ion transport of Li3TMCl6 (TM=Y, Er) and LiF|Li3TMCl6 are investigated using ab initio molecular dynamics and a climbing image nudged elastic band. The results show that the doping of Br-/F- in Li3TMCl6 results in different local anionic coordination in the Li site, which providing a disorder structure for ion transport. The degeneracy and splitting of electron orbitals caused by doping reduce the energy level difference from octahedral to tetrahedral states. The short-range local environment only reduces the migration energy barrier due to the strong electronegativity of F-. However, the broader anionic coordination results in a significant improvement in overall ion transport performance due to the lower electronegativity of Br-. The extremely low migration barrier increases the diffusion coefficient of LiF|Li3TMCl6-X due to weak interactions between transport ions and anions.
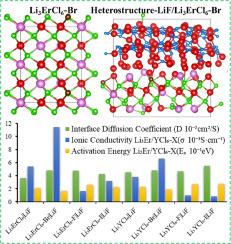
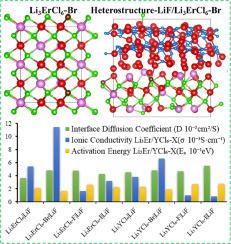
阴离子掺杂对Li3TMCl6 (TM=Y, Er)离子输运的影响及异质结构固体电解质界面第一性原理研究
Li3YCl6和Li3ErCl6因其良好的离子电导率和较宽的稳定化学窗口而被广泛用作全固态电池的固体电解质。Li3YCl6和Li3ErCl6具有三方晶格结构,由于阴离子的电负性较强,其离子传递活化能较高,但与阴极的界面稳定性较差。采用从头算分子动力学和爬升图像轻推弹性带研究了阴离子X (X=F, Br, I)掺杂对Li3TMCl6 (TM=Y, Er)和Li3TMCl6离子输运的影响。结果表明,在Li3TMCl6中掺杂Br-/F-导致Li位点出现不同的局部阴离子配位,为离子传输提供了无序结构。掺杂引起的电子轨道简并和分裂降低了八面体到四面体的能级差。由于F-的强电负性,短距离局部环境只会降低迁移能垒。然而,由于Br-的电负性较低,更广泛的阴离子配位导致了整体离子传输性能的显着改善。由于输运离子和阴离子之间的弱相互作用,极低的迁移势垒增加了LiF|Li3TMCl6-X的扩散系数。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


