Restricting alloying reaction of sns nanoparticles as negative electrode towards building high-performance lithium-ion batteries and capacitors with robust cycling
IF 5.6
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
Abstract
The lithium-ion capacitor (LIC) is a relatively new device that has emerged on the battlefield and can potentially put down the LIBs. These hybrid devices have two different electrode mechanisms (Faradaic and non-Faradaic) that bridge the gap between the LIBs and conventional supercapacitors. Herein, we introduce an appropriate electrode material, tin sulphide, SnS, which could be used as an anode in LIBs and LICs by limiting only an alloy-type reaction. This work explores the possibility of fabricating LIB and LIC with SnS nanoparticles as anode and commercial LiNi0.5Mn1.5O4 and activated carbon (AC) as a cathode under balanced loading conditions. For the case of LIC, prior to the fabrication, the Li/SnS cell is electrochemically pre-lithiated (LixSn + Li2S) and paired with the cathode AC. The LIB and LIC displayed a maximum energy density of 387 and 172 Wh kg–1, respectively, with ultra-long cyclability and capacity retention. The adaptability of these devices to various climatic conditions is validated by testing the full cell at different temperature conditions (–10 to 50 °C). Thus, from all the studies, we discovered that the SnS could be a potential candidate that could replace the graphitic carbon anode in both Li-ion batteries and capacitor configurations.
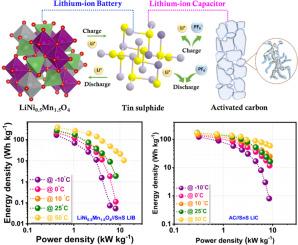
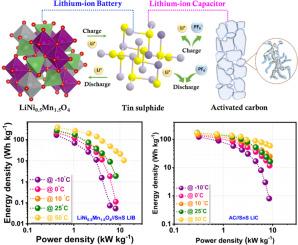
限制SnS纳米负极合金化反应,构建高性能锂离子电池和稳健循环电容器
锂离子电容器(LIC)是一种相对较新的设备,已经出现在战场上,有可能取代锂离子电容器。这些混合装置有两种不同的电极机制(法拉第和非法拉第),弥补了锂离子电池和传统超级电容器之间的差距。在此,我们引入了一种合适的电极材料,硫化锡,SnS,它可以通过限制合金型反应作为锂离子电池和锂离子电池的阳极。本研究探索了在平衡负载条件下,以SnS纳米颗粒为阳极,商用LiNi0.5Mn1.5O4和活性炭(AC)为阴极制备LIB和LIC的可能性。对于锂离子电池,在制造之前,锂/SnS电池被电化学预锂化(LixSn + Li2S)并与阴极AC配对。锂离子电池和锂离子电池的最大能量密度分别为387和172 Wh kg-1,具有超长的可循环性和容量保持性。通过在不同温度条件下(-10至50°C)测试整个电池,验证了这些设备对各种气候条件的适应性。因此,从所有的研究中,我们发现SnS可能是锂离子电池和电容器配置中取代石墨碳阳极的潜在候选者。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


