Dual mechanisms synergistically activating methane and inhibiting overoxidation over ZnO prepared under magnetic field
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
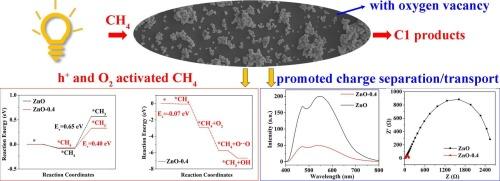
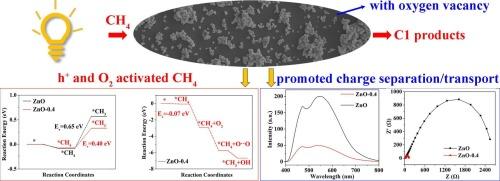
Photocatalytic oxidation of methane (CH4) to value added liquid C1 products offers a promising way for utilizing the abundant CH4 resource, nevertheless, suffering from poor products formation rate and peroxidation. Here, a kind of ZnO-0.4 catalyst with oxygen vacancy (Ov) is prepared under magnetic field for photooxidation CH4 to C1 products with yield rate and selectivity of 13.6 mmol g−1 and 100 %. No peroxide products such as COx were produced even under 8 h of light exposure. Mechanism research indicates that Ov in the catalyst acts as reactive site, facilitates CH4 activation through both h+ and adsorbed-O2 (via formation of Zn–O⋯O–Zn) routes. The two CH4 activation routes proceed simultaneously, which results in promoted charge separation and enhanced catalytic efficiency. This work provides a new approach for the design and preparation of noble metal-free CH4 conversion catalysts.
磁场下制备的氧化锌协同活化甲烷和抑制过氧化的双重机制
甲烷(CH4)光催化氧化制备液态C1增值产品是利用丰富的CH4资源的一种很有前途的方法,但其产物生成速率和过氧化率较低。本文制备了一种氧空位(Ov) ZnO-0.4催化剂,在磁场作用下光氧化CH4生成C1,收率和选择性分别为13.6 mmol g−1和100% %。即使在8 h的光照下,也不会产生COx等过氧化产物。机理研究表明,催化剂中的Ov作为反应位点,通过h+和吸附o2(通过形成Zn-O·O-Zn)两种途径促进CH4活化。两种CH4活化途径同时进行,促进了电荷分离,提高了催化效率。本研究为无贵金属CH4转化催化剂的设计和制备提供了新的途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


