Tumor microenvironment-responsive metal-natural polyphenol nanozyme for radiosensitization by interfering glucose metabolism and redox homeostasis
IF 11.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Radiotherapy (RT) still faces great challenges with tumor hypoxia, antioxidant mechanisms and high glucose metabolism. Herein, we report a tumor microenvironment (TME)-responsive ruthenium-natural polyphenol phloretin (RPP) nanozyme with glucose oxidase (GOx), catalase (CAT), peroxidase (POD) and glutathione peroxidase (GPX) -like activities, which serves as an efficient and safe radiosensitizer for enhanced RT. Under an acidic TME, RPP nanozyme aerobically consumes the intracellular glucose and reduces the production of lactic acid, and meanwhile, the in-situ exposure of phloretin can block the uptake of extracellular glucose by inhibiting glucose transporters, making it more efficient to inhibit glycolysis and minimize oxygen consumption. Moreover, RPP nanozyme can not only rapidly catalyze hydrogen peroxide (H2O2) into reactive oxygen species (ROS) and oxygen to alleviate tumor hypoxia, but also down-regulate the intracellular glutathione (GSH) and lactic acid level to overcome antioxidant metabolism. Both in vitro and in vivo studies manifest that RPP nanozyme significantly enhance the radiosensitivity of 4 T1 tumor cells. Besides, in vivo positron emission tomography imaging, utilizing clinical imaging agents, validates the decreased glucose uptake and hypoxia relief treated by RPP nanozyme. The facile RPP nanozyme with the regulating ability of TME can achieve TME-responsive radiosensitization, providing promising potential for tumor therapy.
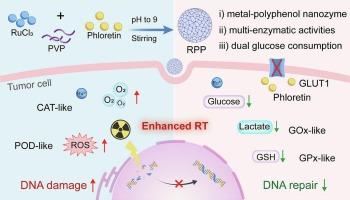
肿瘤微环境响应金属-天然多酚纳米酶通过干扰葡萄糖代谢和氧化还原稳态实现放射增敏
放疗仍面临着肿瘤缺氧、抗氧化机制和高糖代谢的巨大挑战。本文报道了一种具有葡萄糖氧化酶(GOx)、过氧化氢酶(CAT)、过氧化物酶(POD)和谷胱甘肽过氧化物酶(GPX)样活性的肿瘤微环境(TME)响应钌-天然多酚根黄素(RPP)纳米酶,作为一种高效、安全的增强rt的放射增敏剂。在酸性TME下,RPP纳米酶有氧消耗细胞内葡萄糖并减少乳酸的产生,同时,根皮素的原位暴露可以通过抑制葡萄糖转运体来阻断细胞外葡萄糖的摄取,从而更有效地抑制糖酵解和减少氧气消耗。此外,RPP纳米酶不仅可以快速催化过氧化氢(H2O2)转化为活性氧(ROS)和氧气,缓解肿瘤缺氧,还可以下调细胞内谷胱甘肽(GSH)和乳酸水平,克服抗氧化代谢。体外和体内研究均表明,RPP纳米酶可显著增强4 T1肿瘤细胞的放射敏感性。此外,体内正电子发射断层成像,利用临床显像剂,验证了RPP纳米酶治疗葡萄糖摄取减少和缺氧缓解。具有TME调节能力的易降解RPP纳米酶可实现TME应答性放射致敏,为肿瘤治疗提供了广阔的前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


