Synthesis and antibacterial evaluation of 5-(3-nitrophenyl)-N′-arylisoxazole-3-carbohydrazide derivatives against carbapenem-resistant Acinetobacter baumannii
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
The gram-negative coccobacillus Acinetobacter baumannii, a WHO critical priority pathogen and member of ESKAPE pathogen panel, threatens global health due to its substantial association with hospital-acquired infections as well as resistance exhibited to last-resort antibiotics, such as colistin, tigecycline and carbapenems. Thus, urgent drug discovery and development is essential, targeting Acinetobacter baumannii, especially carbapenem-resistant Acinetobacter baumannii (CRAB). In this context, we report the design, synthesis and anti-bacterial evaluation of a new collection of 5-(phenyl)-N′-arylisoxazole-3-carbohydrazides for their effectiveness against various bacterial pathogen panels. Amongst the various compounds synthesised, 7j, 7l, 7n, 7o, 7p, and 16 exhibited significant antibacterial activity against CRAB, with minimum inhibitory concentrations (MIC) 0.5–2 μg/mL. Compound 7l demonstrated the highest antibacterial effectiveness, with a 0.5 μg/mL MIC. Additional testing revealed that these compounds were non-toxic to Vero cells and displayed high selectivity indices. Furthermore, they were effective against clinical isolates of multidrug-resistant Acinetobacter baumannii (MDR-AB), with compound 7l showing bactericidal effects when paired with Rifampicin, as supported by time-kill kinetic studies. 3D QSAR confirms 7ls increased activity comes from good steric fit, ideal electrostatics, strategic hydrophobic placement, and precise H-bond acceptor/donor positioning, and 7l complies with Lipinski's rule of five. The molecular target for these compounds is not known, though in silico α-fold modelling indicate that KatG may be the probable target. However in-depth mechanistic studies need to be done to validate these in silico predictions, based on which 7l may be further optimised as a promising candidate targeting CRAB.
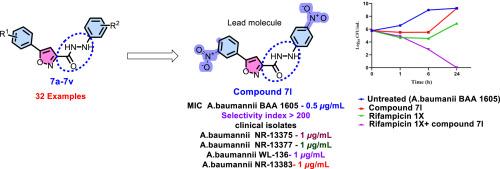
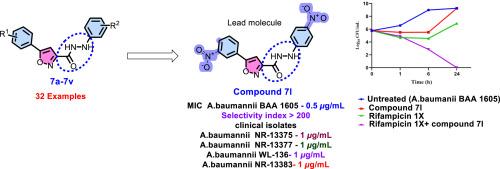
5-(3-硝基苯基)- n′-芳基异恶唑-3-碳肼衍生物的合成及对耐碳青霉烯鲍曼不动杆菌的抗菌评价
鲍曼不动杆菌是世卫组织的重要重点病原体,也是ESKAPE病原体小组的成员,由于与医院获得性感染存在重大关联,以及对粘菌素、地加环素和碳青霉烯类等最后手段抗生素表现出耐药性,威胁着全球健康。因此,迫切需要针对鲍曼不动杆菌,特别是耐碳青霉烯类鲍曼不动杆菌(CRAB)进行药物研发。在此背景下,我们报道了一种新的5-(苯基)- n '-芳基异恶唑-3-碳腙类化合物的设计、合成和抗菌评价,以证明它们对各种细菌病原体的有效性。在所合成的化合物中,7j、7l、7n、70、7p和16对螃蟹具有显著的抑菌活性,最小抑菌浓度(MIC)为0.5 ~ 2 μg/mL。化合物7l抗菌效果最好,MIC为0.5 μg/mL。进一步的测试表明,这些化合物对Vero细胞无毒,并表现出高选择性指数。此外,它们对临床分离的多药耐药鲍曼不动杆菌(MDR-AB)有效,化合物7l与利福平配对时显示出杀菌作用,这得到了时间杀伤动力学研究的支持。3D QSAR证实7l活性的增加来自于良好的空间配合、理想的静电、战略性的疏水放置和精确的氢键受体/供体定位,并且7l符合Lipinski的五法则。这些化合物的分子靶标尚不清楚,但硅α-fold模型表明KatG可能是可能的靶标。然而,需要进行深入的机制研究来验证这些计算机预测,在此基础上,7l可能会进一步优化为靶向螃蟹的有希望的候选药物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


