Polyamine crosslinked with diisocyanates: Reprocessing, shape recovery and polyelectrolyte properties
IF 4.5
2区 化学
Q2 POLYMER SCIENCE
引用次数: 0
Abstract
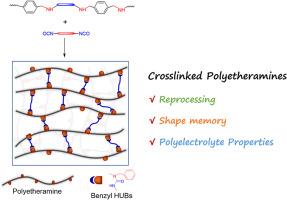
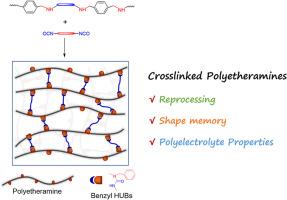
A linear polyamine (PEA) was synthesized and then crosslinked with diisocyanates. The crosslinking led to the integration of benzyl hindered urea bonds (HUBs) into the PEA networks. First, the Schiff base reaction of terephthaldicarboxaldehyde with a polymeric diamine bearing poly(ethylene oxide) (PEO) block was carried out to generate a linear poly(ether imine), which was then transformed into the PEA through hydrogenation reaction. By taking control of types and contents of diisocyanates, the PEA networks were regulated displaying variable thermomechanical properties. More importantly, the PEA networks can be reprocessable (or recyclable); the dynamic exchange of benzyl HUBs was responsible for the excellent reprocessing (recycling) properties. Thanks to the crosslinking, the PEA networks had the shape memory properties featuring the reconfigurability. Benefiting from the integration of PEO blocks, the PEA networks can be converted into the solid polyelectrolytes (SPEs). Incorporated with an electrolyte [e.g., lithium bis(trifluoromethanesulphonyl)imide, LiTFSI], the networks can have the ionic conductivity as high as 7.1 × 10−5 S × cm−1 at 300 K. Notably, the SPEs integrated with benzyl HUBs were recyclable without sacrificing the ionic conductivity.
多胺与二异氰酸酯交联:再加工、形状恢复及聚电解质性能
合成了一种线性多胺(PEA),并与二异氰酸酯交联。交联导致苯基阻碍尿素键整合到PEA网络中。首先,对苯二甲酸二醛与含聚二胺的聚环氧乙烷(PEO)进行希夫碱反应生成线性聚醚亚胺,然后通过加氢反应转化为PEA。通过控制二异氰酸酯的种类和含量,可以调节PEA网络,显示不同的热机械性能。更重要的是,PEA网络可以重新处理(或回收);苯基轮毂的动态交换是其优良的再处理(回收)性能的主要原因。由于交联,PEA网络具有可重构的形状记忆特性。得益于PEO块的集成,PEA网络可以转化为固体聚电解质(spe)。加入电解质[例如,二(三氟甲烷磺基)亚胺,LiTFSI],网络可以在300 K时具有高达7.1 × 10-5 S × cm-1的离子电导率。值得注意的是,与苄基hub集成的spe是可回收的,而不会牺牲离子电导率。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Polymer
化学-高分子科学
CiteScore
7.90
自引率
8.70%
发文量
959
审稿时长
32 days
期刊介绍:
Polymer is an interdisciplinary journal dedicated to publishing innovative and significant advances in Polymer Physics, Chemistry and Technology. We welcome submissions on polymer hybrids, nanocomposites, characterisation and self-assembly. Polymer also publishes work on the technological application of polymers in energy and optoelectronics.
The main scope is covered but not limited to the following core areas:
Polymer Materials
Nanocomposites and hybrid nanomaterials
Polymer blends, films, fibres, networks and porous materials
Physical Characterization
Characterisation, modelling and simulation* of molecular and materials properties in bulk, solution, and thin films
Polymer Engineering
Advanced multiscale processing methods
Polymer Synthesis, Modification and Self-assembly
Including designer polymer architectures, mechanisms and kinetics, and supramolecular polymerization
Technological Applications
Polymers for energy generation and storage
Polymer membranes for separation technology
Polymers for opto- and microelectronics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


