Impact of adolescent ethanol binge on serotonin signaling and pain sensitivity post-withdrawal
IF 2.9
4区 医学
Q3 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Adolescence is a critical neurodevelopmental period characterized by heightened neuroplasticity. While the acute effects of binge ethanol (EtOH) consumption are documented, its long-term impact on both pain sensitivity and microglial activation during adolescence remains unclear. Given serotonin's (5-HT) known involvement in pain processing and sensitivity to EtOH, this study examined the effects of adolescent EtOH binge on microglia-induced neuroinflammation in serotonergic nuclei, downstream 5-HT signaling, and pain sensitivity at different time points after EtOH withdrawal. Adolescent male C57BL/6J mice received triweekly oral gavage of 20 % EtOH or water for 4 weeks and were assessed after 24 h and 3 weeks post-withdrawal. We used immunohistochemistry to assess neuroinflammation in the dorsal raphe, median raphe, and raphe magnus by labeling 5-HT, CD68, and P2Y12. Further analyses examined downstream signaling via 5-HT and serotonin transporter (SERT) expression in the nucleus accumbens, anterior cingulate cortex, thalamus, amygdala, hypothalamus, and raphe magnus. Pain sensitivity was then assessed using the Hargreaves test. EtOH exposure led to widespread serotonergic and neuroinflammatory changes. Significant increases in microglia-induced neuroinflammation were observed in the dorsal raphe nucleus, median raphe nucleus, and raphe magnus nucleus after both 24 h and 3 weeks post-withdrawal, along with significant deficits in 5-HT. Similar 5-HT deficits were observed in downstream regions—notably in the anterior cingulate cortex, thalamus, amygdala, and hypothalamus—at varying time points post-withdrawal. EtOH-exposed mice also showed lasting hyperalgesia at both 24 h and 3 weeks post-withdrawal that persisted for up to 9 weeks. These results suggest that persistent hyperalgesia following adolescent EtOH binge may be driven by changes in serotonergic function and microglial activation.
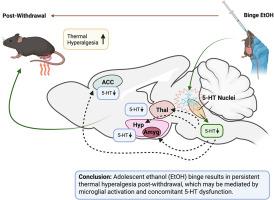
青少年酒精暴饮对戒断后血清素信号传导和疼痛敏感性的影响。
青春期是神经发育的关键时期,其特点是神经可塑性增强。虽然暴饮乙醇(EtOH)的急性效应有文献记载,但其对青春期疼痛敏感性和小胶质细胞激活的长期影响尚不清楚。鉴于已知5-羟色胺(5-HT)参与疼痛加工和对EtOH的敏感性,本研究在EtOH戒断后的不同时间点检测了青少年EtOH暴饮对小胶质细胞诱导的5-羟色胺能核神经炎症、下游5-HT信号传导和疼痛敏感性的影响。青春期雄性C57BL/6J小鼠每3周灌胃20% EtOH或水,连续灌胃4周,停药后24小时和停药后3周进行评估。通过标记5-HT、CD68和P2Y12,我们使用免疫组织化学来评估中缝背、中缝和大缝的神经炎症。进一步的分析检测了伏隔核、前扣带皮层、丘脑、杏仁核、下丘脑和大中脑中通过5-羟色胺和血清素转运体(SERT)表达的下游信号。然后用哈格里夫斯试验评估疼痛敏感性。EtOH暴露导致广泛的血清素能和神经炎症改变。戒断后24小时和3周,中缝背核、中缝中核和中缝大核的小胶质细胞诱导的神经炎症显著增加,同时5-羟色胺明显不足。在戒断后的不同时间点,在下游区域——尤其是前扣带皮层、丘脑、杏仁核和下丘脑——也观察到类似的5-羟色胺缺陷。暴露于etoh的小鼠在停药后24小时和3周也表现出持续的痛觉过敏,持续时间长达9周。这些结果表明,青春期EtOH暴食后的持续性痛觉过敏可能是由血清素能功能和小胶质细胞激活的变化驱动的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Alcohol
医学-毒理学
CiteScore
4.60
自引率
4.30%
发文量
74
审稿时长
15.6 weeks
期刊介绍:
Alcohol is an international, peer-reviewed journal that is devoted to publishing multi-disciplinary biomedical research on all aspects of the actions or effects of alcohol on the nervous system or on other organ systems. Emphasis is given to studies into the causes and consequences of alcohol abuse and alcoholism, and biomedical aspects of diagnosis, etiology, treatment or prevention of alcohol-related health effects.
Intended for both research scientists and practicing clinicians, the journal publishes original research on the neurobiological, neurobehavioral, and pathophysiological processes associated with alcohol drinking, alcohol abuse, alcohol-seeking behavior, tolerance, dependence, withdrawal, protracted abstinence, and relapse. In addition, the journal reports studies on the effects alcohol on brain mechanisms of neuroplasticity over the life span, biological factors associated with adolescent alcohol abuse, pharmacotherapeutic strategies in the treatment of alcoholism, biological and biochemical markers of alcohol abuse and alcoholism, pathological effects of uncontrolled drinking, biomedical and molecular factors in the effects on liver, immune system, and other organ systems, and biomedical aspects of fetal alcohol spectrum disorder including mechanisms of damage, diagnosis and early detection, treatment, and prevention. Articles are published from all levels of biomedical inquiry, including the following: molecular and cellular studies of alcohol''s actions in vitro and in vivo; animal model studies of genetic, pharmacological, behavioral, developmental or pathophysiological aspects of alcohol; human studies of genetic, behavioral, cognitive, neuroimaging, or pathological aspects of alcohol drinking; clinical studies of diagnosis (including dual diagnosis), treatment, prevention, and epidemiology. The journal will publish 9 issues per year; the accepted abbreviation for Alcohol for bibliographic citation is Alcohol.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


