Research advancements on sumoylation in gastrointestinal cancers
IF 10.3
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
SUMOylation is a critical post-translational modification that modulates protein activity, stability, and subcellular distribution through the covalent attachment of SUMO proteins (SUMO1-5) to specific targets. This process is mediated by a cascade of enzymes, including E1, E2, E3 ligases, and deSUMOylation enzymes, enabling precise control over diverse biological functions such as gene expression, cell cycle regulation, DNA damage repair, signaling cascades, and metabolic pathways.
Dysregulation of SUMOylation enzymes has been contributes to cancer initiation, and treatment resistance, by enhancing tumor cell motility, aggressiveness, and epithelial-mesenchymal transition (EMT). In gastrointestinal malignancies—including gastric, hepatic, colorectal, esophageal, gallbladder, and pancreatic cancers—SUMOylation drives tumor growth, metastasis, and invasiveness by reprogramming metabolic processes, signaling networks, and the surrounding tumor niche. Additionally, it contributes to resistance against chemotherapy and radiotherapy.
Understanding the molecular basis of SUMOylation not only underscores its significance in oncogenesis but also provides a foundation for developing novel anticancer therapies.
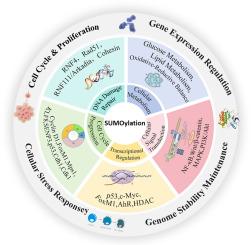
胃肠道肿瘤中sumoylation的研究进展
SUMO蛋白(SUMO1-5)与特定靶标的共价附着调节蛋白活性、稳定性和亚细胞分布,是一种重要的翻译后修饰。该过程由一系列酶介导,包括E1、E2、E3连接酶和deSUMOylation酶,从而精确控制多种生物功能,如基因表达、细胞周期调节、DNA损伤修复、信号级联和代谢途径。sumo酰化酶的失调通过增强肿瘤细胞的运动性、侵袭性和上皮-间质转化(EMT),有助于癌症的发生和治疗抵抗。在胃肠道恶性肿瘤中,包括胃癌、肝癌、结肠直肠癌、食管癌、胆囊癌和胰腺癌,sumoylation通过重编程代谢过程、信号网络和周围肿瘤生态位驱动肿瘤生长、转移和侵袭性。此外,它有助于抵抗化疗和放疗。了解SUMOylation的分子基础不仅强调了其在肿瘤发生中的重要性,而且为开发新的抗癌疗法提供了基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Aspects of Medicine
医学-生化与分子生物学
CiteScore
18.20
自引率
0.00%
发文量
85
审稿时长
55 days
期刊介绍:
Molecular Aspects of Medicine is a review journal that serves as an official publication of the International Union of Biochemistry and Molecular Biology. It caters to physicians and biomedical scientists and aims to bridge the gap between these two fields. The journal encourages practicing clinical scientists to contribute by providing extended reviews on the molecular aspects of a specific medical field. These articles are written in a way that appeals to both doctors who may struggle with basic science and basic scientists who may have limited awareness of clinical practice issues. The journal covers a wide range of medical topics to showcase the molecular insights gained from basic science and highlight the challenging problems that medicine presents to the scientific community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


