Sn-2 palmitate attenuates neuroinflammation in aged mice via sex-specific modulation of the microbiota-gut-brain axis
引用次数: 0
Abstract
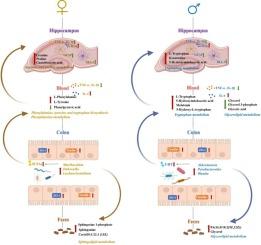
Previous studies have demonstrated that sn-2 palmitate promotes neurodevelopment in early-life hosts (e.g., infants and newly weaned mice). However, its neuroprotective effects in aged mice and the underlying mechanisms remain unclear. In this study, we employed 18-month-old C57BL/6 mice as an aging model. After six months of dietary supplementation with sn-2 palmitate at varying concentrations (47.62 %, 59.15 % or 75.79 %), both male and female aged mice exhibited attenuated neuroinflammation accompanied by the reduced expression of glial activation markers (IBA-1 and GFAP). Notably, this neuroprotective effect was mediated by regulating the microbiota-gut-brain axis in a sex-dependent manner and was most pronounced in aged mice supplemented with 75.79 % sn-2 palmitate. In females, it enriched gut microbiota belonging to the genera Lachnoclostridium, Dubosiella, and Muribaculum, up-regulated phenylalanine, tyrosine and tryptophan biosynthesis, and phenylalanine metabolism pathways in serum and enhanced fecal sphingolipid excretion, collectively reducing systemic inflammation. Meanwhile, the up-regulation of arginine and proline metabolism in the hippocampus enhanced its energy buffering capacity and stress resilience. In males, sn-2 palmitate increased the abundances of Akkermansia, Parabacteroides, and Blautia genera, which enhanced tryptophan metabolism and elevated its serum derivatives, while reducing circulating glycerolipid levels and promoting their fecal excretion. The tryptophan-glycerolipid metabolic regulation exerted a dual anti-inflammatory effect, suppressing systemic inflammation and directly attenuating neuroinflammation. Together, our findings provide novel insights into the neuroprotective potential of dietary sn-2 palmitate in aged models and establish a foundation for developing precision dietary interventions tailored to sex differences, with potential implications for improving brain health in aging populations.
Sn-2棕榈酸酯通过对微生物-肠-脑轴的性别特异性调节减轻老年小鼠的神经炎症
先前的研究表明,sn-2棕榈酸酯促进早期宿主(如婴儿和刚断奶的小鼠)的神经发育。然而,其对老年小鼠的神经保护作用及其机制尚不清楚。本研究采用18月龄C57BL/6小鼠作为衰老模型。在饮食中添加不同浓度(47.62 %,59.15 %或75.79 %)的n-2棕榈酸酯6个月后,雄性和雌性老年小鼠均表现出神经炎症减轻,同时神经胶质激活标志物(IBA-1和GFAP)的表达减少。值得注意的是,这种神经保护作用是通过以性别依赖的方式调节微生物-肠-脑轴介导的,在添加75.79 % sn-2棕榈酸酯的老年小鼠中最为明显。在雌性中,它丰富了Lachnoclostridium、Dubosiella和Muribaculum属的肠道微生物群,上调了苯丙氨酸、酪氨酸和色氨酸的生物合成以及血清中苯丙氨酸的代谢途径,增强了粪便鞘脂的排泄,共同减少了全身性炎症。同时,海马体内精氨酸和脯氨酸代谢上调,增强了其能量缓冲能力和应激恢复能力。在雄性中,n-2棕榈酸酯增加了Akkermansia、parabobacteroides和Blautia属的丰度,从而增强了色氨酸代谢并提高了其血清衍生物,同时降低了循环甘油脂水平并促进了它们的粪便排泄。色氨酸-甘油脂代谢调节具有抑制全身炎症和直接减轻神经炎症的双重抗炎作用。总之,我们的研究结果为老年模型中sn-2棕榈酸盐饮食的神经保护潜力提供了新的见解,并为开发针对性别差异的精确饮食干预奠定了基础,对改善老年人的大脑健康具有潜在的意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


