Assisted therapy optimizing module to improve physician adhErence with guideLine-directed medical heart failure therapy rationale and design of the AMPEL trial
IF 2.5
Q2 CARDIAC & CARDIOVASCULAR SYSTEMS
引用次数: 0
Abstract
Background
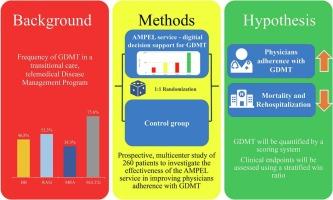
Guideline-directed medical therapy (GDMT) is very effective in the early, vulnerable phase after discharge following an acute heart failure (HF) event, but its widespread implementation in everyday clinical practice is limited.
AMPEL is a guideline-based decision support software service for physicians to improve adherence with GDMT. The four drug classes that make up the GDMT and the percentage of each prescribed dose in relation to the target dose according to the current ESC guidelines are displayed graphically. A daily update considers the current therapy as well as absolute or relative contraindications.
Methods and results
We present the rationale and protocol of the AMPEL trial, a multicenter randomized trial with a parallel-group design to investigate the effectiveness of the AMPEL service on GDMT implementation and patient clinical outcomes in a total of 260 patients. Salient features of the AMPEL study are that it is being conducted as part of the established disease management program (DMP) for HF, HerzMobil, and that participating physicians are given randomised access to the AMPEL service compared to the standard of care in the control group. The primary endpoint includes an implementation endpoint for assessment of the quality of indication- and dose-adjusted GDMT at 90 days and a clinical endpoint assessed with a stratified win ratio.
Conclusions
The AMPEL trial is a prospective, randomized trial investigating the effectiveness of a guideline-based decision support software service for physicians to improve adherence with GDMT in a telemedicine-assisted disease management program for patients with heart failure.
辅助治疗优化模块以提高医生对指南指导的医学心力衰竭治疗的依从性AMPEL试验的基本原理和设计
背景:指南导向的药物治疗(GDMT)在急性心力衰竭(HF)事件后出院后的早期脆弱阶段非常有效,但其在日常临床实践中的广泛实施受到限制。AMPEL是一种基于指南的决策支持软件服务,可帮助医生提高GDMT的依从性。构成GDMT的四种药物类别以及根据当前ESC指南,每种处方剂量相对于目标剂量的百分比以图形方式显示。每日更新考虑当前治疗以及绝对或相对禁忌症。方法和结果我们介绍了AMPEL试验的基本原理和方案,这是一项多中心随机试验,采用平行组设计,旨在调查AMPEL服务对GDMT实施和患者临床结果的有效性,共有260例患者。AMPEL研究的显著特点是,它是作为HF HerzMobil既定疾病管理计划(DMP)的一部分进行的,并且参与的医生被随机分配到AMPEL服务,与对照组的标准护理相比。主要终点包括一个用于评估90天适应症和剂量调整GDMT质量的实施终点,以及一个用分层胜比评估的临床终点。AMPEL试验是一项前瞻性随机试验,旨在调查基于指南的决策支持软件服务在远程医疗辅助心衰患者疾病管理项目中提高GDMT依从性的有效性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
IJC Heart and Vasculature
Medicine-Cardiology and Cardiovascular Medicine
CiteScore
4.90
自引率
10.30%
发文量
216
审稿时长
56 days
期刊介绍:
IJC Heart & Vasculature is an online-only, open-access journal dedicated to publishing original articles and reviews (also Editorials and Letters to the Editor) which report on structural and functional cardiovascular pathology, with an emphasis on imaging and disease pathophysiology. Articles must be authentic, educational, clinically relevant, and original in their content and scientific approach. IJC Heart & Vasculature requires the highest standards of scientific integrity in order to promote reliable, reproducible and verifiable research findings. All authors are advised to consult the Principles of Ethical Publishing in the International Journal of Cardiology before submitting a manuscript. Submission of a manuscript to this journal gives the publisher the right to publish that paper if it is accepted. Manuscripts may be edited to improve clarity and expression.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


