Inclisiran in a real-world single-center registry of patients with very high atherosclerotic cardiovascular risk
IF 2.1
Q3 PERIPHERAL VASCULAR DISEASE
引用次数: 0
Abstract
Background
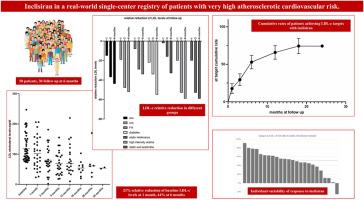
A mean relative 50 % reduction of LDL cholesterol (LDLc) levels was observed in randomized studies in patients treated with inclisiran, a small-interfering-RNA therapeutic agent that reduces hepatic synthesis of PCSK9. Less is known on real world and every-day practice patients.
Methods
Fifty consecutive patients with or at risk of atherosclerotic cardiovascular disease (ASCVD) or familial hypercholesterolemia (FH) and treated with inclisiran according to Italian indications were enrolled in an observational study. LDLc levels were followed up.
Results
26 patients had an acute coronary syndrome (ACS), 11 FH; 46 % were on high-intensity statin therapy, 68 % on combination therapy statin/ezetimibe.
Mean LDLc level of the study population was 118 ± 12 mg/dl at baseline, 80 ± 18 mg/dl after 3 months, and 70 ± 15 mg/dl after 6 months (ANOVA p < 0.001). The use of inclisiran was associated with significantly reduced LDLc levels of 21 % at 1 month and 44 % at 6 months.
LDLc reduction in patients with ACS was statistically significant and comparable with chronic CS. Patients receiving a background combination therapy (statin/ezetimibe) showed a greater reduction in circulating LDLc levels than patients using inclisiran alone. No significant side effects or treatment drop out were observed during follow up. Rates of subjects with LDLc levels below 70 mg/dl (Italian Drug Agency target) increased from 0 % at baseline to 56 % at 6 months (p < 0.001).
Conclusions
In a real-world population 3–6 months of therapy with inclisiran provide consistent and effective reduction in LDLc levels without significant adverse side-effects.
在一个真实世界的单中心注册的患者非常高的动脉粥样硬化性心血管风险
在随机研究中,接受inclisiran治疗的患者LDL胆固醇(LDLc)水平平均相对降低50%。inclisiran是一种小干扰rna治疗药物,可减少肝脏PCSK9的合成。对现实世界和日常实践患者的了解较少。方法:连续50例动脉粥样硬化性心血管疾病(ASCVD)或家族性高胆固醇血症(FH)患者,并根据意大利适应症接受inclisiran治疗,纳入观察性研究。跟踪ldl水平。结果急性冠脉综合征(ACS) 26例,FH 11例;46%接受高强度他汀类药物治疗,68%接受他汀/依折麦布联合治疗。研究人群的平均ldl水平基线时为118±12 mg/dl, 3个月后为80±18 mg/dl, 6个月后为70±15 mg/dl(方差分析p <; 0.001)。使用inclisiran可显著降低ldl水平,1个月时降低21%,6个月时降低44%。ACS患者ldl降低具有统计学意义,与慢性CS相当。接受背景联合治疗(他汀/依折麦布)的患者比单独使用inclisiran的患者循环ldl水平下降更大。随访期间未观察到明显的副作用或治疗退出。ldl水平低于70 mg/dl(意大利药品管理局目标)的受试者比率从基线时的0%增加到6个月时的56% (p < 0.001)。结论:在现实世界人群中,使用inclisiran治疗3-6个月可以持续有效地降低ldl水平,且没有明显的不良副作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Atherosclerosis plus
Cardiology and Cardiovascular Medicine
CiteScore
2.60
自引率
0.00%
发文量
0
审稿时长
66 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


