Efficient removal of methyl orange from water using biofunctional chitosan–cellulose composite: Experimental and DFT insights
IF 5.9
3区 工程技术
Q1 CHEMISTRY, MULTIDISCIPLINARY
Journal of Industrial and Engineering Chemistry
Pub Date : 2025-04-14
DOI:10.1016/j.jiec.2025.04.030
引用次数: 0
Abstract
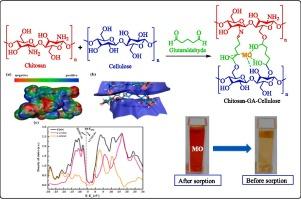
In this study, a biofunctional composite, chitosan–GA–cellulose composite (CGAC), was synthesized through a cross-linking process involving chitosan and cellulose, using glutaraldehyde (GA) as the cross-linking agent. The final composite material was tested for the removal of the pollutant methylene orange dye. The structural and chemical properties of the carbohydrate-based composite were investigated using several techniques, including XRD, SEM, EDX, TGA and FTIR. The results show that the synthesized material has good textural properties and high temperature resistance. Equilibrium results were well described by the Langmuir isotherm. Under optimal conditions, specifically, an initial MO concentration of 100 mg/L, a contact time of 80 min, and a pH of 4.4, the maximum removal efficiency of MO reached 98.9 %, with an adsorption capacity of 324.62 mg/g. Even after five regeneration cycles, the removal efficiency remained high at 83.4 %, demonstrating the adsorbent’s strong reusability. To further understand the interfacial interactions and adsorption behavior of methyl orange (MO) on the synthesized CGAC composite, density functional theory (DFT) calculations were employed. These theoretical investigations provided a comprehensive understanding of the adsorption mechanism by elucidating the electronic properties, structural formation, and fundamental bonding interactions between MO and CGAC. The DFT analysis revealed critical insights into charge distribution, donor–acceptor interactions, and adsorption energy, offering a deeper perspective on the stability and efficiency of MO removal by CGAC material. The as-prepared carbohydrate-based composite exhibits significant potential for wastewater treatment because of its high adsorption efficiency, environmentally friendly composition, and sustainable nature.
利用生物功能壳聚糖-纤维素复合材料从水中有效去除甲基橙:实验和DFT见解
本研究以戊二醛(GA)为交联剂,以壳聚糖和纤维素为原料,通过交联法制备了壳聚糖- GA -纤维素复合材料(CGAC)。对最终的复合材料进行了去除污染物亚甲基橙染料的试验。采用XRD、SEM、EDX、TGA和FTIR等技术对碳水化合物基复合材料的结构和化学性质进行了研究。结果表明,合成材料具有良好的织构性能和耐高温性能。Langmuir等温线很好地描述了平衡结果。在MO初始浓度为100 mg/L、接触时间为80 min、pH为4.4的最佳条件下,MO的最大去除率达到98.9%,吸附量为324.62 mg/g。即使经过5次再生循环,吸附剂的去除率仍高达83.4%,表明吸附剂具有较强的可重复使用性。为了进一步了解甲基橙(MO)在合成的CGAC复合材料上的界面相互作用和吸附行为,采用密度泛函理论(DFT)计算。这些理论研究通过阐明MO和CGAC之间的电子性质、结构形成和基本键相互作用,为全面了解其吸附机理提供了依据。DFT分析揭示了电荷分布,供体-受体相互作用和吸附能的关键见解,为CGAC材料去除MO的稳定性和效率提供了更深入的视角。所制备的碳水化合物基复合材料由于其高吸附效率、环境友好性和可持续性,在废水处理方面具有巨大的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.40
自引率
6.60%
发文量
639
审稿时长
29 days
期刊介绍:
Journal of Industrial and Engineering Chemistry is published monthly in English by the Korean Society of Industrial and Engineering Chemistry. JIEC brings together multidisciplinary interests in one journal and is to disseminate information on all aspects of research and development in industrial and engineering chemistry. Contributions in the form of research articles, short communications, notes and reviews are considered for publication. The editors welcome original contributions that have not been and are not to be published elsewhere. Instruction to authors and a manuscript submissions form are printed at the end of each issue. Bulk reprints of individual articles can be ordered. This publication is partially supported by Korea Research Foundation and the Korean Federation of Science and Technology Societies.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


