One-step facile synthesis of Ba2V2O7 nanostructures for electrochemical behaviour improvement of symmetric supercapacitor
IF 5.9
3区 工程技术
Q1 CHEMISTRY, MULTIDISCIPLINARY
Journal of Industrial and Engineering Chemistry
Pub Date : 2025-05-03
DOI:10.1016/j.jiec.2025.05.001
引用次数: 0
Abstract
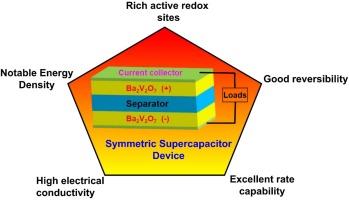
In order to create energy storage systems that are quicker, more dependable and more secure, researchers are still developing new materials with extremely high performance. An easy chemical method followed by calcination is employed to create the Ba2V2O7 nanostructure. This electrode has a significant capacitance of 417F/g and stable cycling with 90.6% capacitance retention even after 6000 Galvanostatic Charge-Discharge (GCD) cycles at 1A/g, which exhibits exceptional electrochemical features. These nanostructures are used to develop devices with greater functionality by serving the positive electrode and negative electrode in Ba2V2O7 symmetric configuration. The as-fabricated Ba2V2O7 electrode delivered the notable energy and power densities of 53.3 Wh/kg and 750 W/kg with 88 % optimistic cycle life even after 4000 cycles. The significant electrode electrochemical behaviour is due to the synergistic impacts caused by the electrode components and its unique composite network architecture, which is necessary to provide vital conductivity, rich redox behaviour, quick transfer of electrons, fast dynamics and beneficial active sites for electrochemical reactions. This research aims to provide an advanced platform for efficient and unique electrodes for cutting-edge energy storing devices and small gadgets.
一步合成改善对称超级电容器电化学性能的Ba2V2O7纳米结构
为了创造更快、更可靠、更安全的储能系统,研究人员仍在开发具有极高性能的新材料。采用简单的化学焙烧法制备了Ba2V2O7纳米结构。该电极具有417F/g的显著电容量,在1A/g的恒流充放电(GCD)循环6000次后仍保持90.6%的稳定电容量,表现出优异的电化学特性。这些纳米结构通过在Ba2V2O7对称结构中服务于正极和负极,用于开发具有更大功能的器件。制备的Ba2V2O7电极的能量和功率密度分别为53.3 Wh/kg和750 W/kg,即使在4000次循环后也有88%的乐观循环寿命。重要的电极电化学行为是由于电极组分的协同作用及其独特的复合网络结构,这是提供重要的电导率、丰富的氧化还原行为、快速的电子转移、快速的动力学和有益的电化学反应活性位点所必需的。这项研究旨在为尖端储能设备和小型设备提供高效独特的电极的先进平台。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.40
自引率
6.60%
发文量
639
审稿时长
29 days
期刊介绍:
Journal of Industrial and Engineering Chemistry is published monthly in English by the Korean Society of Industrial and Engineering Chemistry. JIEC brings together multidisciplinary interests in one journal and is to disseminate information on all aspects of research and development in industrial and engineering chemistry. Contributions in the form of research articles, short communications, notes and reviews are considered for publication. The editors welcome original contributions that have not been and are not to be published elsewhere. Instruction to authors and a manuscript submissions form are printed at the end of each issue. Bulk reprints of individual articles can be ordered. This publication is partially supported by Korea Research Foundation and the Korean Federation of Science and Technology Societies.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


