Endolysosomal dysfunction impairs proteostasis and induces neurodegeneration in vivo
IF 4.1
2区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Transactive response (TAR) DNA-binding protein 43 (TDP-43) inclusions are a pathological hallmark of the frontotemporal dementia (FTD)-amyotrophic lateral sclerosis (ALS) spectrum. Dysfunction of the endolysosomal system, which plays a crucial role in protein trafficking and maintaining proteostasis, has been implicated in FTD-ALS pathogenesis. While the impact of endolysosomal dysfunction on TDP-43 pathology remains unclear, we demonstrated that disrupting the endolysosomal pathway by expressing the constitutively active endosomal protein, Rab5Q79L, induces TDP-43 aggregation in cultured cells. Here, we generated a mouse model expressing GFP-tagged Rab5Q79L, demonstrating that GFP-Rab5Q79L mice exhibit early motor deficits and endolysosomal dysfunction, including enlarged endosomes, abnormal lysosome morphology, and p62- or ubiquitin-positive inclusions. These mice also developed significant neuronal loss, neuroinflammation, phosphorylated TDP-43 (pTDP-43) inclusions, and nuclear envelope and nuclear pore structural defects reminiscent of FTD-ALS. Accordingly, GFP-Rab5Q79L mice will prove useful in expanding our understanding of endolysosomal dysfunction in proteostasis and pTDP-43 pathology.
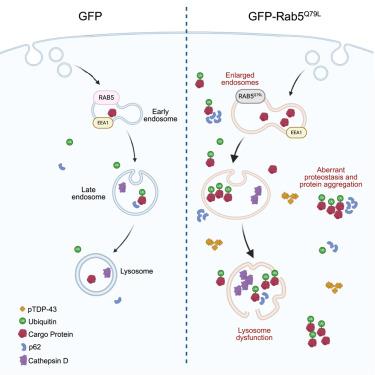
体内内溶酶体功能障碍损害蛋白质平衡并诱导神经退行性变
交互反应(TAR) dna结合蛋白43 (TDP-43)包涵体是额颞叶痴呆(FTD)-肌萎缩侧索硬化症(ALS)谱系的病理标志。内溶酶体系统在蛋白质运输和维持蛋白质稳态中起着至关重要的作用,其功能障碍与FTD-ALS的发病机制有关。虽然内溶酶体功能障碍对TDP-43病理的影响尚不清楚,但我们证明,通过表达构成活性的内溶酶体蛋白Rab5Q79L来破坏内溶酶体途径,可诱导培养细胞中的TDP-43聚集。在这里,我们建立了一个表达gfp标记的Rab5Q79L的小鼠模型,证明GFP-Rab5Q79L小鼠表现出早期运动缺陷和内溶酶体功能障碍,包括内体增大,溶酶体形态异常,p62或泛素阳性包涵体。这些小鼠还出现了显著的神经元丢失、神经炎症、磷酸化的TDP-43 (pTDP-43)内含物,以及令人联想到FTD-ALS的核膜和核孔结构缺陷。因此,GFP-Rab5Q79L小鼠将被证明有助于扩大我们对蛋白酶抑制和pTDP-43病理中内溶酶体功能障碍的理解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

iScience
Multidisciplinary-Multidisciplinary
CiteScore
7.20
自引率
1.70%
发文量
1972
审稿时长
6 weeks
期刊介绍:
Science has many big remaining questions. To address them, we will need to work collaboratively and across disciplines. The goal of iScience is to help fuel that type of interdisciplinary thinking. iScience is a new open-access journal from Cell Press that provides a platform for original research in the life, physical, and earth sciences. The primary criterion for publication in iScience is a significant contribution to a relevant field combined with robust results and underlying methodology. The advances appearing in iScience include both fundamental and applied investigations across this interdisciplinary range of topic areas. To support transparency in scientific investigation, we are happy to consider replication studies and papers that describe negative results.
We know you want your work to be published quickly and to be widely visible within your community and beyond. With the strong international reputation of Cell Press behind it, publication in iScience will help your work garner the attention and recognition it merits. Like all Cell Press journals, iScience prioritizes rapid publication. Our editorial team pays special attention to high-quality author service and to efficient, clear-cut decisions based on the information available within the manuscript. iScience taps into the expertise across Cell Press journals and selected partners to inform our editorial decisions and help publish your science in a timely and seamless way.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


