Plasma-enabled methane upgrading in a gas-liquid reactor: Optimizing a one-step methanol production process
IF 5.9
3区 工程技术
Q1 CHEMISTRY, MULTIDISCIPLINARY
Journal of Industrial and Engineering Chemistry
Pub Date : 2025-04-22
DOI:10.1016/j.jiec.2025.04.038
引用次数: 0
Abstract
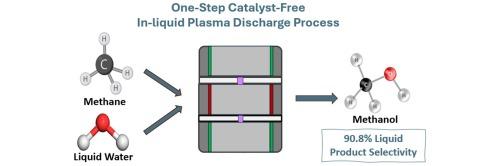
In this work, methane (CH4) and ubiquitous water (H2O) were exploited as reactants in a continuous flow, catalyst-free, nonthermal plasma process to produce methanol at a rate of 21.28 mg/h with a specific yield of 1.46 mgMeOH/gCH4 and selectivity of 90.8 % among other liquid products. A systematic investigation of process parameters through factorial design screened five process factors, i.e., applied power, gas flow rate, liquid flow rate, catalyst loading, and pH. While catalyst loading and pH showed minimal significance, gas flow rate, liquid flow rate, and applied power emerged as the significant factors affecting both production rate and specific yield, though with competing effects where higher gas flow rates enhanced production rates but reduced specific yields. Subsequent optimization using Box-Behnken design determined optimal conditions of 368 W applied power, 273 mL/min methane flow rate, and 51 mL/min water flow rate for maximizing methanol production rate while maintaining high selectivity. OES and NMR analyses revealed a radical-mediated pathway primarily involving methyl and hydroxyl radical coupling for methanol formation. This catalyst-free process showed great promise for cleaner fuel production, reduced greenhouse gas emissions, and efficient utilization of natural gas and biogas resources.
气液反应器中的等离子体甲烷升级:一步甲醇生产过程的优化
在本研究中,利用甲烷(CH4)和水(H2O)作为反应物,在连续流动、无催化剂、非热等离子体工艺中以21.28 mg/h的速率生产甲醇,比产率为1.46 mg/ gCH4,在其他液体产物中选择性为90.8%。通过析因设计对工艺参数进行了系统的调查,筛选出5个工艺因素,即施加功率、气体流量、液体流量、催化剂负载和pH。催化剂负载和pH的影响最小,而气体流量、液体流量和施加功率是影响生产率和比产率的重要因素,尽管存在竞争效应,较高的气体流量提高了生产率,但降低了比产率。随后采用Box-Behnken设计进行优化,确定了368 W功率、273 mL/min甲烷流速和51 mL/min水流速的最佳条件,以最大限度地提高甲醇的产量,同时保持高选择性。OES和NMR分析揭示了一个自由基介导的途径,主要涉及甲基和羟基自由基偶联甲醇的形成。这种无催化剂工艺在清洁燃料生产、减少温室气体排放以及有效利用天然气和沼气资源方面显示出巨大的前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.40
自引率
6.60%
发文量
639
审稿时长
29 days
期刊介绍:
Journal of Industrial and Engineering Chemistry is published monthly in English by the Korean Society of Industrial and Engineering Chemistry. JIEC brings together multidisciplinary interests in one journal and is to disseminate information on all aspects of research and development in industrial and engineering chemistry. Contributions in the form of research articles, short communications, notes and reviews are considered for publication. The editors welcome original contributions that have not been and are not to be published elsewhere. Instruction to authors and a manuscript submissions form are printed at the end of each issue. Bulk reprints of individual articles can be ordered. This publication is partially supported by Korea Research Foundation and the Korean Federation of Science and Technology Societies.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


