Ru and S co-modification-induced synergistic morphology and electronic engineering of nickel-iron hydroxide with efficient oxygen evolution
IF 8.9
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Synchronously achieving morphological and electronic engineering control is crucial but challenging for enhancing the oxygen evolution reaction (OER) performance of nickel-iron based catalysts. Herein, a ruthenium and sulfur co-modified nickel-iron hydroxide (SARuT-FeNiOHx-5h) was synthesized by a distributed room-temperature impregnation method. It was found that the solubility product difference between ruthenium and nickel-iron hydroxide can promote the rapid nucleation of the catalyst and form finer nanosheet structures, thereby increasing 1.25 times for the contact area between the catalyst and the electrolyte. Meanwhile, the subsequent deposition of sulfur can act as an electronic modulator, promoting the transfer of surface charge at nickel sites and increasing the oxidation state of nickel. Theoretical calculations indicate that the combination of ruthenium and sulfur can effectively optimize the OER reaction pathway and lower the activation energy barrier of the rate-determining step, endowing SARuT-FeNiOHx-5h an excellent OER performance with a low overpotential of 253 mV at 1000 mA/cm2 and long-term stability (500h). In the future, it is hoped that this strategy of synergistic control of morphology and electronic structure can be applied to the development of other highly active catalysts.
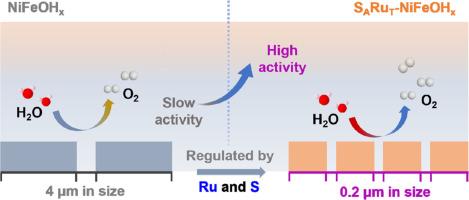
Ru和S共改性诱导高效析氧氧化镍铁的协同形貌和电子工程
同时实现形态和电子工程控制对于提高镍铁基催化剂的析氧反应性能至关重要,但具有挑战性。本文采用分布式室温浸渍法制备了钌硫共改性氢氧化镍铁(SARuT-FeNiOHx-5h)。结果表明,钌与氢氧化镍铁的溶解度积差异能促进催化剂的快速成核,形成更精细的纳米片结构,从而使催化剂与电解质的接触面积增加1.25倍。同时,随后的硫沉积可以起到电子调制器的作用,促进镍位点表面电荷的转移,提高镍的氧化态。理论计算表明,钌和硫的结合可以有效地优化OER反应途径,降低速率决定步骤的活化能垒,使SARuT-FeNiOHx-5h具有优异的OER性能,在1000 mA/cm2下过电位为253 mV,长期稳定(500h)。在未来,希望这种协同控制形貌和电子结构的策略可以应用于其他高活性催化剂的开发。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chinese Chemical Letters
化学-化学综合
CiteScore
14.10
自引率
15.40%
发文量
8969
审稿时长
1.6 months
期刊介绍:
Chinese Chemical Letters (CCL) (ISSN 1001-8417) was founded in July 1990. The journal publishes preliminary accounts in the whole field of chemistry, including inorganic chemistry, organic chemistry, analytical chemistry, physical chemistry, polymer chemistry, applied chemistry, etc.Chinese Chemical Letters does not accept articles previously published or scheduled to be published. To verify originality, your article may be checked by the originality detection service CrossCheck.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


