The study of natural dolomite as a prospective material for CO2 capture employing a novel approach to the evaluation of breakthrough curves
IF 2.8
Q1 MATERIALS SCIENCE, CERAMICS
引用次数: 0
Abstract
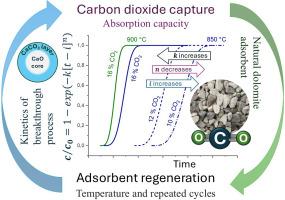
The capture of carbon dioxide (CO2) is a critical technology for addressing climate change and sustainability objectives. In this study, the performance of natural dolomite as an effective sorbent for repeated CO2 capture was evaluated. The results suggested that an optimal calcination temperature of 850 °C was beneficial for minimizing surface sintering of the dolomite, thereby facilitating effective decarbonation. Breakthrough curve analysis was conducted to evaluate the dynamic adsorption performance of dolomite at varying CO2 concentrations (10 %, 12 %, and 16 %). To assess the progress of gas adsorption onto regenerated dolomite, an innovative method of curve fitting using the modified Avrami equation was employed, which provided three essential parameters for the adsorption process: retention time, rate constant, and Avrami coefficient. A steady decrease in breakthrough time and adsorption efficiency was found to be correlated with sintering and surface area loss. The maximum CO2 adsorption capacity was achieved during the second or third cycle for all three measured CO2 concentrations; however, performance gradually deteriorated in subsequent cycles due to surface sintering and a reduction in specific surface area. TPD and BET analyses supported the conclusion that the surface area decreased with repeated regeneration, and the basic active sites were reduced.
采用一种新的方法来评估突破曲线,研究天然白云岩作为二氧化碳捕获的有前景的材料
二氧化碳捕集是应对气候变化和实现可持续发展目标的关键技术。在这项研究中,天然白云石作为一种有效的吸附剂,反复捕获二氧化碳的性能进行了评估。结果表明,850℃的最佳焙烧温度有利于减少白云石的表面烧结,从而促进有效的脱碳。通过突破曲线分析,评价了不同CO2浓度(10%、12%和16%)下白云石的动态吸附性能。为了评估再生白云岩上气体吸附的进展,采用了一种基于修正Avrami方程的创新曲线拟合方法,该方法提供了吸附过程的三个基本参数:保留时间、速率常数和Avrami系数。突破时间和吸附效率的稳定下降与烧结和表面积损失有关。在第二次或第三次循环中,所有三种测量的CO2浓度均达到最大CO2吸附量;然而,在随后的循环中,由于表面烧结和比表面积的减少,性能逐渐恶化。TPD和BET分析表明,随着再生的不断进行,其表面积减小,基本活性位点减少。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Open Ceramics
Materials Science-Materials Chemistry
CiteScore
4.20
自引率
0.00%
发文量
102
审稿时长
67 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


