Hydrothermal synthesis and growth of stable strontium apatite for radioactive salt waste immobilization
IF 3.5
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
Safe management of chloride-rich radioactive salt waste produced during molten-salt dry reprocessing of spent nuclear fuel is essential for the sustainable deployment of advanced nuclear energy systems. Although 90SrCl2 represents only a minor mass fraction, its high specific activity and decay heat dominate short-term thermal management. In this work, a low temperature hydrothermal synthesis of Sr5(PO4)3Cl effectively immobilized Sr and about 10 % of Cl, while the remaining Cl was released as NaCl. Over a 10-h hydrothermal process, the step-like growth of crystalline and partially disordered domains promotes enhanced crystallinity and increased particle size of the Sr5(PO4)3Cl solidified form, thereby improving its chemical durability. The well-crystallized product exhibits a mass loss of less than 1.8 wt.% at 1000 °C, and normalized leach rate of Sr2+ is 3.5 × 10−6 gm−2d−1 after 28 d in deionized water at 90 °C. Reliable thermal and chemical stability confirm that low-temperature hydrothermal processing is an energy-efficient strategy for conditioning 90SrCl2 chloride wastes prior to geological disposal.
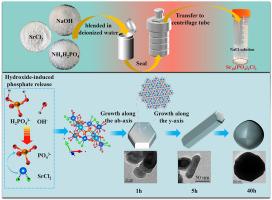
稳定磷灰石锶的水热合成与生长
对乏核燃料熔盐干式后处理过程中产生的富氯放射性盐废物进行安全管理,对于先进核能系统的可持续部署至关重要。虽然90SrCl2只占很小的质量分数,但其高比活度和衰变热主导了短期热管理。本文采用低温水热法合成Sr5(PO4)3Cl,有效地固定了Sr和约10%的Cl,剩余的Cl以NaCl的形式释放。在10 h的水热过程中,晶体和部分无序结构域的阶梯状生长促进了Sr5(PO4)3Cl固化形态的结晶度增强和粒径增大,从而提高了其化学耐久性。结晶良好的产物在1000℃下的质量损失小于1.8 wt.%,在90℃的去离子水中28 d后,Sr2+的归一化浸出率为3.5 × 10−6 gm−2d−1。可靠的热稳定性和化学稳定性证实,低温水热处理是在地质处置前对90SrCl2氯化废物进行处理的节能策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Solid State Chemistry
化学-无机化学与核化学
CiteScore
6.00
自引率
9.10%
发文量
848
审稿时长
25 days
期刊介绍:
Covering major developments in the field of solid state chemistry and related areas such as ceramics and amorphous materials, the Journal of Solid State Chemistry features studies of chemical, structural, thermodynamic, electronic, magnetic, and optical properties and processes in solids.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


