Structural and optical properties of ZnO and Cu-doped ZnO thin films and the influence of surface defects on glucose oxidation
IF 3.4
4区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Zinc oxide (ZnO) and 4 % copper-doped ZnO (Cu–ZnO) thin films were synthesized by the co-precipitation method, deposited via spin-coating, and annealed at 450 °C for 6 h. Structural and surface analyses using X-ray diffraction (XRD), scanning electron microscopy (SEM), atomic force microscopy (AFM), and energy-dispersive X-ray spectroscopy (EDX) confirmed enhanced crystallinity and grain growth, with crystallite size increasing from ∼17 to ∼31 nm and surface roughness from 2.8 to 8.3 nm after Cu doping and annealing. Photoluminescence (PL) spectra revealed suppressed oxygen-vacancy–related emission, consistent with reduced defect levels and improved crystallinity. Glucose oxidation, investigated by attenuated total reflectance Fourier transform infrared (ATR-FTIR) spectroscopy, showed that oxygen vacancies dominate in unannealed ZnO, whereas surface copper oxide (CuO) species drive oxidation in annealed Cu–ZnO through Cu2+/Cu3+ redox reactions. These results demonstrate that defect engineering combined with low-Cu-content doping in thin films effectively enhances glucose oxidation, offering a simple yet effective strategy for ZnO-based non-enzymatic glucose sensors.
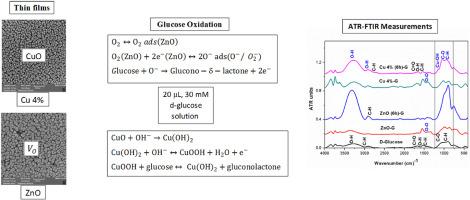
ZnO和cu掺杂ZnO薄膜的结构和光学性质及表面缺陷对葡萄糖氧化的影响
采用共沉淀法合成氧化锌(ZnO)和4%铜掺杂ZnO (Cu-ZnO)薄膜,通过自旋镀膜沉积,并在450℃下退火6 h。利用x射线衍射(XRD)、扫描电子显微镜(SEM)、原子力显微镜(AFM)和能量色散x射线能谱(EDX)对其结构和表面进行分析,证实其结晶度和晶粒生长得到了增强。Cu掺杂和退火后,晶粒尺寸从~ 17 nm增加到~ 31 nm,表面粗糙度从2.8 nm增加到8.3 nm。光致发光(PL)光谱显示与氧空位相关的发射受到抑制,与缺陷水平降低和结晶度提高一致。利用衰减全反射傅里叶变换红外光谱(ATR-FTIR)研究了葡萄糖氧化,发现未退火ZnO中主要是氧空位,而表面氧化铜(CuO)通过Cu2+/Cu3+氧化还原反应驱动氧化。这些结果表明,缺陷工程与薄膜中低cu含量掺杂的结合有效地增强了葡萄糖的氧化,为基于zno的非酶葡萄糖传感器提供了一种简单而有效的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
3.50
自引率
7.70%
发文量
492
审稿时长
3-8 weeks
期刊介绍:
The Journal of the Indian Chemical Society publishes original, fundamental, theorical, experimental research work of highest quality in all areas of chemistry, biochemistry, medicinal chemistry, electrochemistry, agrochemistry, chemical engineering and technology, food chemistry, environmental chemistry, etc.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


