Mononuclear phagocytes in blood and cerebrospinal fluid of patients with relapsing-remitting multiple sclerosis: Untreated and treated with anti-CD20 therapy
IF 2.5
4区 医学
Q3 IMMUNOLOGY
引用次数: 0
Abstract
Background
Mononuclear phagocytes, including monocytes, macrophages, and microglia, play key roles in the immunopathogenesis of multiple sclerosis (MS). While anti-CD20 monoclonal antibody therapies effectively treat relapsing-remitting MS (RRMS), their secondary effects on mononuclear phagocytes remain unclear.
Objective and methods
We analyzed blood and cerebrospinal fluid (CSF) mononuclear phagocytes in patients with RRMS treated with anti-CD20 therapy for 6 months, untreated patients, and controls. Flow cytometry was used to assess the prevalence, phenotype, and cytokine production by blood monocytes. Chitinase-1 (CHIT1) levels in CSF were measured using an enzyme-linked immunosorbent assay.
Results
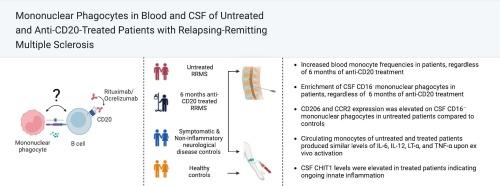
Blood monocyte frequencies were elevated in untreated and anti-CD20-treated RRMS patients compared with controls. CSF mononuclear phagocytes differed from blood monocytes and were classified as either CD16+ or CD16−, with CD16+ mononuclear phagocytes being enriched in all groups. However, the frequencies of CD16− mononuclear phagocytes in CSF were higher in untreated and anti-CD20-treated RRMS patients compared to controls, and the expression of CD206 and CCR2 on CD16− mononuclear phagocytes differed between untreated patients and controls. Blood monocyte cytokine production did not differ between untreated and anti-CD20-treated patients. CSF CHIT1 levels were elevated in both untreated and anti-CD20-treated patients.
Conclusions
The comparable blood and CSF mononuclear phagocyte phenotypes, along with persistently elevated CHIT1 concentrations in CSF of untreated and anti-CD20-treated patients with RRMS, suggest that residual innate immune activation is not normalized by 6 months of anti-CD20 treatment.
复发-缓解型多发性硬化症患者血液和脑脊液中的单核吞噬细胞:未经治疗和抗cd20治疗
单核吞噬细胞,包括单核细胞、巨噬细胞和小胶质细胞,在多发性硬化症(MS)的免疫发病机制中起着关键作用。虽然抗cd20单克隆抗体治疗可有效治疗复发缓解型MS (RRMS),但其对单核吞噬细胞的继发性作用尚不清楚。目的和方法分析抗cd20治疗6个月的RRMS患者、未治疗患者和对照组的血液和脑脊液(CSF)单核吞噬细胞。流式细胞术用于评估血液单核细胞的患病率、表型和细胞因子的产生。采用酶联免疫吸附法测定脑脊液几丁质酶-1 (CHIT1)水平。结果与对照组相比,未经治疗和抗cd20治疗的RRMS患者血液单核细胞频率升高。脑脊液单核吞噬细胞与血液单核细胞不同,分为CD16+和CD16 -两种,CD16+单核吞噬细胞在所有组中均富集。然而,与对照组相比,未经治疗和抗cd20治疗的RRMS患者脑脊液中CD16 -单核吞噬细胞的频率更高,CD206和CCR2在CD16 -单核吞噬细胞上的表达在未经治疗的患者和对照组之间存在差异。未治疗和抗cd20治疗的患者血液单核细胞因子的产生没有差异。未经治疗和抗cd20治疗的患者脑脊液CHIT1水平均升高。结论:未治疗和抗cd20治疗的RRMS患者的血液和脑脊液单核吞噬细胞表型相似,脑脊液中CHIT1浓度持续升高,表明抗cd20治疗6个月后,残余的先天免疫激活未恢复正常。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of neuroimmunology
医学-免疫学
CiteScore
6.10
自引率
3.00%
发文量
154
审稿时长
37 days
期刊介绍:
The Journal of Neuroimmunology affords a forum for the publication of works applying immunologic methodology to the furtherance of the neurological sciences. Studies on all branches of the neurosciences, particularly fundamental and applied neurobiology, neurology, neuropathology, neurochemistry, neurovirology, neuroendocrinology, neuromuscular research, neuropharmacology and psychology, which involve either immunologic methodology (e.g. immunocytochemistry) or fundamental immunology (e.g. antibody and lymphocyte assays), are considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


