A dual-functional in situ hydrogel for delivering vitamin E–based lipid nanoparticles to enhance cancer immunotherapy
IF 10.2
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Conventional mRNA lipid nanoparticles often fail to elicit robust antitumor immunity due to their limited capacity to overcome the immunosuppressive tumor microenvironment (TME). Rational design of ionizable lipids with intrinsic bioactivity presents a promising strategy to enhance mRNA-based cancer immunotherapy. Here, we synthesized a bioactive vitamin E–based ionizable lipid to formulate lipid nanoparticles co-loaded with IL-12 mRNA and the IDO1 inhibitor NLG919 (N@VEBLNP), which were subsequently embedded into polyether F127-diacrylate hydrogel (NVF Gel). This hydrogel enables thermosensitive gelation for intratumoral injection and photocrosslinkable curing for postoperative site retention in the treatment of triple-negative breast cancer (TNBC). Specifically, NVF Gel exerted a dual immunomodulatory function: sustained release of N@VEBLNPs activated migratory cDC1s and augmented antigen presentation in tumor-draining lymph nodes, while concomitant release of NLG919 inhibited IDO1 expression, reduced regulatory T cells, and reprogrammed M2 macrophages toward the M1 phenotype. In 4T1 murine models, NVF Gel transformed the tumor environment into a more “immune-hot” state, effectively suppressed tumor growth and delayed postoperative recurrence. Collectively, NVF Gel provides a versatile platform for in situ cancer immunization and tumor microenvironment modulation.
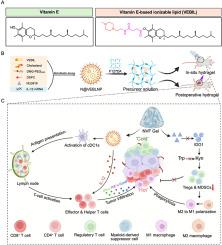
一种双重功能的原位水凝胶,用于输送维生素基脂质纳米颗粒,以增强癌症免疫治疗
传统的mRNA脂质纳米颗粒由于其克服免疫抑制肿瘤微环境(TME)的能力有限,通常无法引发强大的抗肿瘤免疫。合理设计具有内在生物活性的可电离脂质为增强基于mrna的癌症免疫治疗提供了一种有前途的策略。在这里,我们合成了一种具有生物活性的维生素基可电离脂质,以制备脂质纳米颗粒,共负载IL-12 mRNA和IDO1抑制剂NLG919 (N@VEBLNP),随后将其嵌入聚醚f127 -二丙烯酸酯水凝胶(NVF凝胶)中。这种水凝胶可用于肿瘤内注射的热敏凝胶和用于三阴性乳腺癌(TNBC)治疗的术后部位保留的光交联固化。具体来说,NVF凝胶具有双重免疫调节功能:持续释放N@VEBLNPs活化的迁移cDC1s并增强肿瘤引流淋巴结中的抗原呈递,同时释放NLG919抑制IDO1表达,减少调节性T细胞,并将M2巨噬细胞重编程为M1表型。在4T1小鼠模型中,NVF凝胶将肿瘤环境转变为更“免疫热”的状态,有效抑制肿瘤生长,延迟术后复发。总的来说,NVF凝胶为原位癌症免疫和肿瘤微环境调节提供了一个通用的平台。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


