Clinically friendly smart hydrogel boosts cuproptosis and PD-L1 upregulation to enhance anti-tumor immunotherapy
IF 10.2
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Triple-negative breast cancer (TNBC) faces the challenge of limited treatment efficacy due to its highly invasive ability and its immunosuppressive microenvironment. This study found that cuproptosis, as an emerging therapeutic strategy, has unique therapeutic potential in TNBC. Nevertheless, the delivery difficulties of cuproptosis-inducing agents and the limited efficacy of single drugs restrict the clinical application of cuproptosis therapy. Herein, a temperature/pH dual-responsive composite hydrogel was developed to load Elesclomol-Cu (ES-Cu) and glucose oxidase (GOx) (ES-Cu&GOx@FFC). ES and Cu2+ can synergistically trigger cuproptosis in TNBC, and GOx can not only inhibit tumor metabolism by mediating glucose deprivation but also initiate the Fenton reaction by continuously generating H2O2 and synergizing with copper ions, driving a potent chemodynamic therapy (CDT). Furthermore, ES-Cu&GOx@FFC could significantly improve the immunosuppressive landscape and upregulate programmed death-ligand 1 (PD-L1) expression in TNBC. Combined therapy experiments showed that the combination treatment of ES-Cu&GOx@FFC and αPD-L1 achieved more than 90 % tumor volume regression. In summary, this study provides new insights into the therapeutic role of cuproptosis in TNBC, and integrates cuproptosis, starvation therapy, CDT, and immunotherapy through smart responsive hydrogels, providing an innovative solution for the treatment of TNBC.
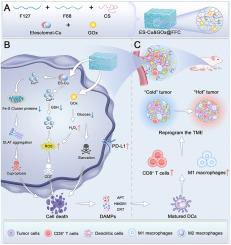
临床友好型智能水凝胶促进铜增生和PD-L1上调,增强抗肿瘤免疫治疗
三阴性乳腺癌(triple negative breast cancer, TNBC)侵袭性强,微环境免疫抑制,治疗效果有限。本研究发现,铜瓣置换术作为一种新兴的治疗策略,在TNBC中具有独特的治疗潜力。然而,铜倾诱导药物的递送困难和单一药物的疗效有限,限制了铜倾治疗的临床应用。本研究开发了一种温度/pH双响应复合水凝胶,用于加载埃来氯莫酚- cu (ES-Cu)和葡萄糖氧化酶(ES-Cu&GOx@FFC)。ES和Cu2+可以协同触发TNBC中的cuprotosis,而GOx不仅可以通过介导葡萄糖剥夺来抑制肿瘤代谢,还可以通过持续产生H2O2并与铜离子协同启动Fenton反应,驱动有效的化学动力学治疗(CDT)。此外,ES-Cu&;GOx@FFC可以显著改善TNBC的免疫抑制状况,上调程序性死亡配体1 (PD-L1)的表达。联合治疗实验表明,ES-Cu&;GOx@FFC与αPD-L1联合治疗可使肿瘤体积缩小90%以上。综上所述,本研究对铜质增生在TNBC中的治疗作用提供了新的见解,并通过智能反应性水凝胶将铜质增生、饥饿治疗、CDT和免疫治疗相结合,为TNBC的治疗提供了创新的解决方案。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


