Simultaneous induction of immunogenic cell death and PD-L1 downregulation by bufalin-based nanovaccines for potentiate HCC immunotherapy
IF 10.2
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Immunotherapy is a promising treatment in hepatocellular carcinoma (HCC), but with low response rate clinically. Immunogenic cell death (ICD) is considered as a strategy to enhance immunotherapy response. However, chemotherapeutic drugs with ICD make tumor cells upregulating PD-L1 expression to deactivate T-cells via PD-1/PD-L1 pathway. Here, Bufalin (Buf) was validated for the first time as an ICD inducer. Buf triggered reactive oxygen species (ROS) related endoplasmic reticulum (ER) stress to elicit apoptosis and ICD via PERK/eIF2α/ATF-4/CHOP in HCC cells. Additionally, Buf downregulated the expression of PD-L1 to avoid immune escape. Buf can simultaneously activate dendritic cell (DC) maturation and interrupt the PD-1/PD-L1 pathway. To amplify immunotherapy and decrease adverse cardiac reactions of Buf, SP94-modified liposome-coated zeolite imidazolate framework-8 loaded with Buf (Buf-ZIF-lipo-SP94) was designed to effectively increase drug accumulation in tumor by HCC-specific targeting SP94 receptor-mediated endocytosis. Importantly, ZIF-8 with the ability of triggering ROS generation itself synergized Buf to induce stronger ICD effects. Buf-ZIF-lipo-SP94 achieved synergistic effects with anti-PD-L1 for HCC immunotherapy, showing better tumor inhibition rate (>90 %), survival of animals and safety. This study uncovered the potential of Buf in inducing ICD and downregulating PD-L1 expression, developing a Buf-loaded nanovaccine for combination strategy of immunotherapy in HCC.
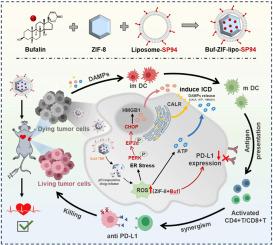
以蟾毒灵为基础的纳米疫苗同时诱导免疫原性细胞死亡和PD-L1下调以增强HCC免疫治疗
免疫疗法是治疗肝细胞癌的一种很有前景的治疗方法,但临床应答率低。免疫原性细胞死亡(ICD)被认为是增强免疫治疗反应的一种策略。而ICD化疗药物通过PD-1/PD-L1通路,使肿瘤细胞上调PD-L1表达,使t细胞失活。在这里,bufain (Buf)首次被验证为ICD诱导剂。但触发活性氧(ROS)相关的内质网(ER)应激,通过PERK/eIF2α/ATF-4/CHOP诱导HCC细胞凋亡和ICD。此外,Buf下调PD-L1的表达以避免免疫逃逸。但它可以同时激活树突状细胞(DC)成熟并中断PD-1/PD-L1通路。为了扩大免疫治疗并减少Buf的心脏不良反应,设计了装载Buf的SP94修饰脂质体包被咪唑酸分子筛框架-8 (Buf- zif - lipop -SP94),通过hcc特异性靶向SP94受体介导的内吞作用,有效增加肿瘤内药物积累。重要的是,ZIF-8与触发ROS生成的能力本身协同作用,但诱导更强的ICD效应。但在HCC免疫治疗中,f- zif - lipop - sp94与抗pd - l1具有协同作用,具有更好的肿瘤抑制率(90%)、动物存活率和安全性。本研究揭示了Buf在诱导ICD和下调PD-L1表达方面的潜力,开发了一种负载Buf的纳米疫苗,用于HCC免疫治疗的联合策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


