Engineered thioglycolate-activated carbon composites via ambient alcohol esterification for enhanced mercury(II) adsorption performance: The role of alcohols in thioglycolic acid esterification
IF 6.7
2区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
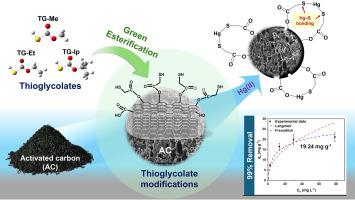
Mercury contamination necessitates effective remediation strategies. This study synthesized thioglycolate-modified activated carbon (TG/AC) via facile, eco-friendly esterification of thioglycolic acid (TGA) using methanol, ethanol, and isopropanol under ambient conditions as a sustainable alternative to harsh methods. The materials were evaluated for their ability to remove Hg(II) from contaminated water at pH 6. Methanol-derived 8 %Me-TG/AC demonstrated exceptional performance, removing 99 % of Hg(II) within 20 min (1.0 mg g−1 adsorption capacity), significantly outperforming TGA(DI)/AC control and other alcohol-derived variants. This superior efficiency is attributed to methanol facilitating a uniform distribution of thiol active sites, leading to a highly negative surface charge that promotes both favorable electrostatic attraction and strong Hg![]() S chemisorption. Advanced characterization via X-ray photoelectron spectroscopy (XPS), X-ray absorption spectroscopy (XAS), and density functional theory (DFT) calculations confirmed direct, covalent Hg
S chemisorption. Advanced characterization via X-ray photoelectron spectroscopy (XPS), X-ray absorption spectroscopy (XAS), and density functional theory (DFT) calculations confirmed direct, covalent Hg![]() S bond formation as the primary adsorption mechanism, with high calculated binding energies. Comparing 8 %Me-TG/AC to 4 %Me-TG/AC mechanistically revealed that higher thiol loading creates a more defined and uniform Hg
S bond formation as the primary adsorption mechanism, with high calculated binding energies. Comparing 8 %Me-TG/AC to 4 %Me-TG/AC mechanistically revealed that higher thiol loading creates a more defined and uniform Hg![]() S coordination environment (dominated by species such as Hg3S2Cl2), enhancing removal efficiency over lower loadings and TGA(DI)/AC control. This work provides key insights for advancing the understanding of thioglycolate preparation mechanisms on AC surfaces for environmental mercury remediation.
S coordination environment (dominated by species such as Hg3S2Cl2), enhancing removal efficiency over lower loadings and TGA(DI)/AC control. This work provides key insights for advancing the understanding of thioglycolate preparation mechanisms on AC surfaces for environmental mercury remediation.
巯基乙酸酯-活性炭复合材料环境酒精酯化增强汞(II)吸附性能:酒精在巯基乙酸酯化中的作用
汞污染需要有效的补救策略。本研究以甲醇、乙醇和异丙醇为原料,在环境条件下通过巯基乙酸(TGA)的酯化反应合成巯基乙酸改性活性炭(TG/AC),以替代苛刻的酯化方法。评估了这些材料在pH为6的污染水中去除汞(II)的能力。甲醇衍生的8% Me-TG/AC表现出优异的性能,在20分钟内去除99%的Hg(II) (1.0 mg g - 1的吸附量),显著优于TGA(DI)/AC对照和其他醇衍生的变体。这种优越的效率归因于甲醇促进了硫醇活性位点的均匀分布,导致高度负的表面电荷,促进了有利的静电吸引力和强HgS化学吸附。通过x射线光电子能谱(XPS)、x射线吸收能谱(XAS)和密度泛函理论(DFT)计算的高级表征证实,直接、共价的HgS键形成是主要的吸附机制,具有较高的计算结合能。比较8% Me-TG/AC和4% Me-TG/AC的机制发现,较高的硫醇负荷创造了一个更明确和均匀的HgS配位环境(由Hg3S2Cl2等物种主导),比低负荷和TGA(DI)/AC控制提高了去除效率。这项工作提供了关键的见解,促进理解巯基乙酸酯制备机制在交流表面的环境汞修复。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of water process engineering
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
10.70
自引率
8.60%
发文量
846
审稿时长
24 days
期刊介绍:
The Journal of Water Process Engineering aims to publish refereed, high-quality research papers with significant novelty and impact in all areas of the engineering of water and wastewater processing . Papers on advanced and novel treatment processes and technologies are particularly welcome. The Journal considers papers in areas such as nanotechnology and biotechnology applications in water, novel oxidation and separation processes, membrane processes (except those for desalination) , catalytic processes for the removal of water contaminants, sustainable processes, water reuse and recycling, water use and wastewater minimization, integrated/hybrid technology, process modeling of water treatment and novel treatment processes. Submissions on the subject of adsorbents, including standard measurements of adsorption kinetics and equilibrium will only be considered if there is a genuine case for novelty and contribution, for example highly novel, sustainable adsorbents and their use: papers on activated carbon-type materials derived from natural matter, or surfactant-modified clays and related minerals, would not fulfil this criterion. The Journal particularly welcomes contributions involving environmentally, economically and socially sustainable technology for water treatment, including those which are energy-efficient, with minimal or no chemical consumption, and capable of water recycling and reuse that minimizes the direct disposal of wastewater to the aquatic environment. Papers that describe novel ideas for solving issues related to water quality and availability are also welcome, as are those that show the transfer of techniques from other disciplines. The Journal will consider papers dealing with processes for various water matrices including drinking water (except desalination), domestic, urban and industrial wastewaters, in addition to their residues. It is expected that the journal will be of particular relevance to chemical and process engineers working in the field. The Journal welcomes Full Text papers, Short Communications, State-of-the-Art Reviews and Letters to Editors and Case Studies
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


